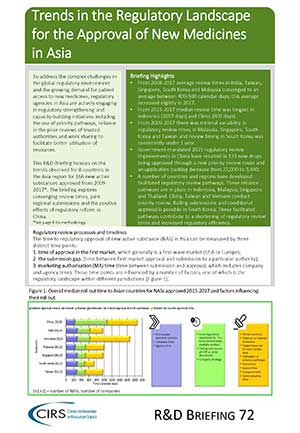
Webinar on approaches to implementing regulatory reliance
We recently presented key findings from our latest R&D Briefing in a webinar on 'Approaches to Implementing Regulatory Reliance: Considerations for Agencies'. The webinar was attended by over 200 people [...]