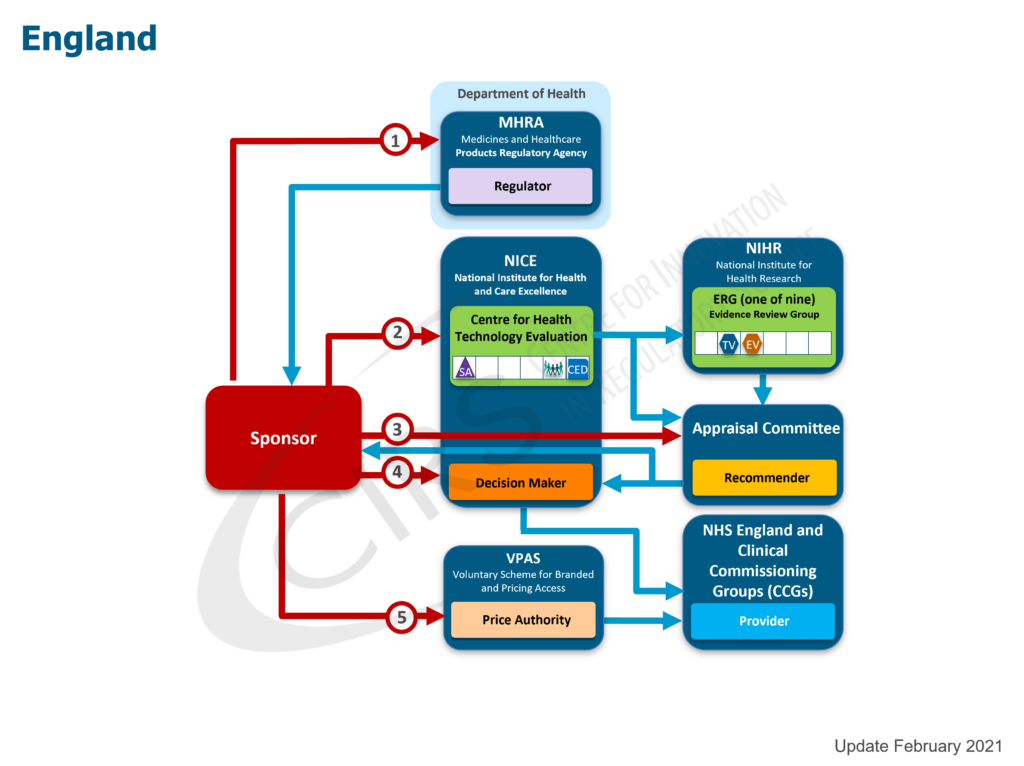
Regulatory and Reimbursement Atlas
The ability to effectively navigate regulatory, HTA and payer pathways throughout the product lifecycle is key to successful medicine development. As the global development environment becomes more complex, the need to understand the confluence of these pathways has become a driver of the medicine development process.
Promoting alignment by mapping regulatory and reimbursement processes
Over the past 20 years, CIRS has collaborated with more than 75 regulatory, HTA and payer agencies to understand the nature of their activities. Using our proprietary methodology to map regulatory and reimbursement pathways and to illustrate the core functions of each agency involved, CIRS has developed a simple, globally recognised approach to understanding this diverse landscape. Thus, the CIRS Regulatory and Reimbursement Atlas™ was developed to illustrate the sequence of interactions with agencies in each jurisdiction, while understanding each agency’s particular functions. More than 70 national and regional maps were created for the following regions: Asia, Europe, North America, Oceania and South America.
Such process mapping allows the planning of development strategy by identifying potentially rate-limiting steps. Benchmarking industry, regulatory and HTA performance against peers with similar mandates and processes can encourage good practices and promote timeliness, predictability, consistency, transparency, clarity, efficiency and quality. Tracking and measuring performance can convey achievements and needs to policy makers, promote continuous improvements and opportunities for work optimisation and build trust in each other’s systems and approaches.
Supporting policy change across HTA agencies
The Atlas was presented at the 6th meeting of the HTA Network (Brussels, 20 May 2016) to demonstrate the comparative mapping method and identification of differences and similarities of HTA systems in Europe. It has also been shared with the EU Commission to support a mapping project that has informed the 2017 Impact Assessment.
Supporting research with HTA agencies
The Atlas outlines which agencies provide scientific advice to companies during drug development, which is currently a muchdiscussed area. CIRS were approached by the Swedish HTA agency, TLV, to undertake an assessment of HTA agencies’ perceptions of the value of providing scientific advice. The results of the study have been presented at international workshops.
Strategic tool for companies
The Atlas is available to the 20+ CIRS member companies to help meet the following goals:
- Plan market access strategy by identifying potentially rate-limiting process steps
- Compare processes between jurisdictions to facilitate simultaneous development programmes and to identify potential ‘best practice’ pathways
- Train staff on the diversity of regulatory and reimbursement systems to inform strategic planning for evidence generation and deliver value messages to different stakeholders.