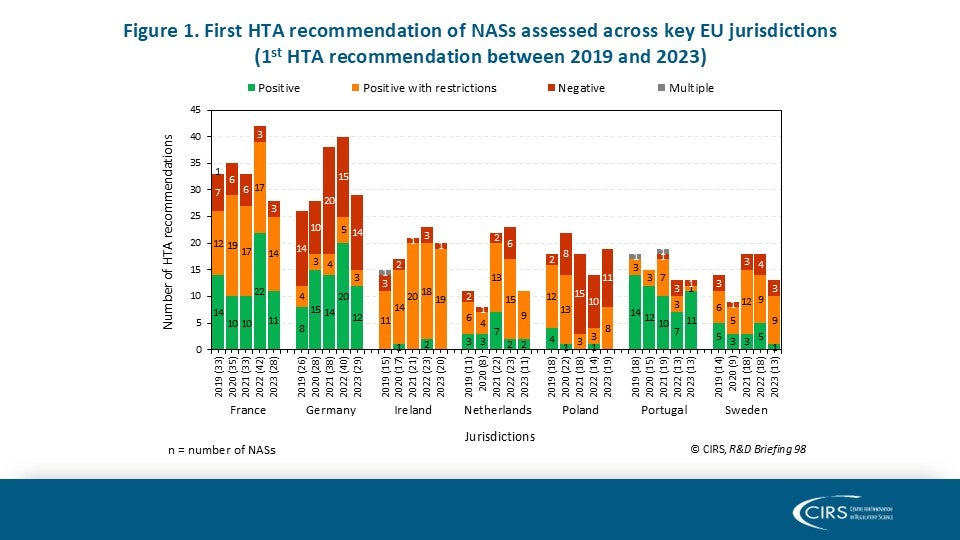
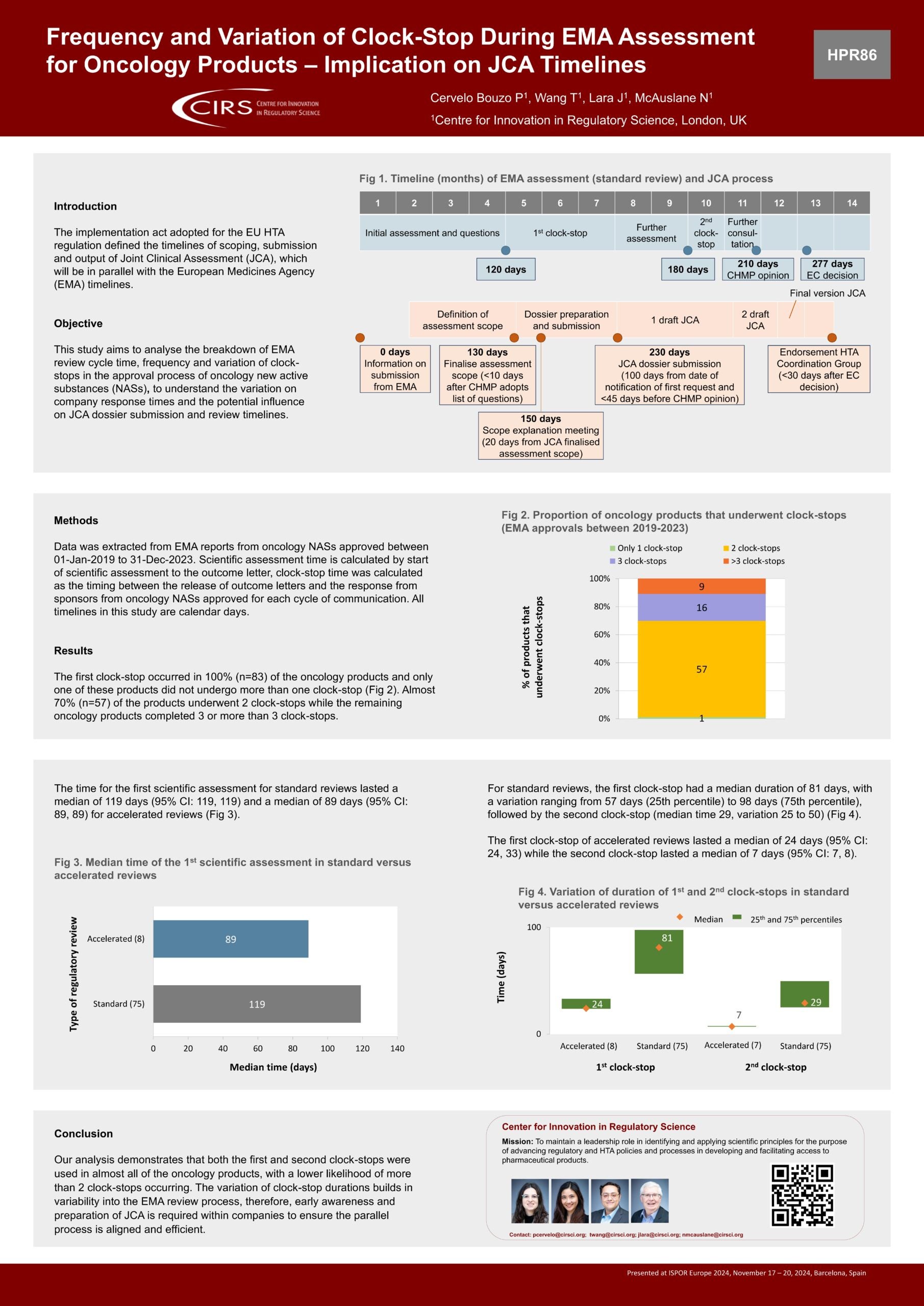
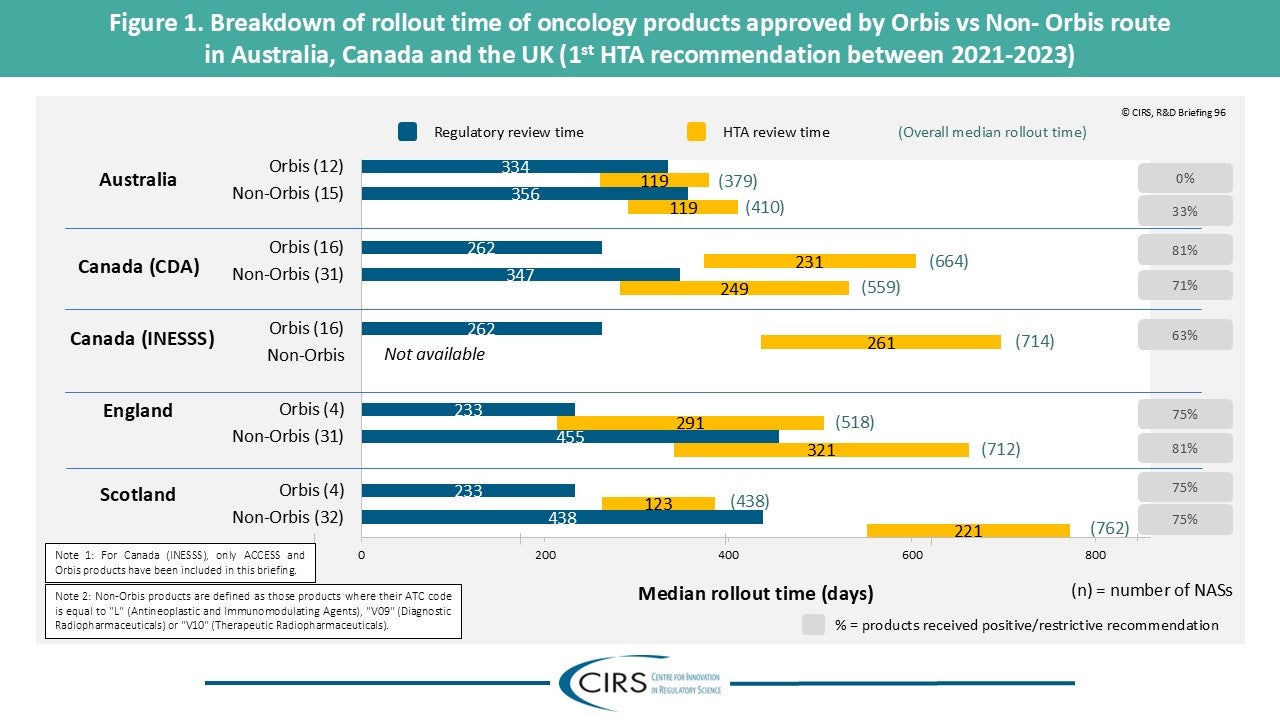
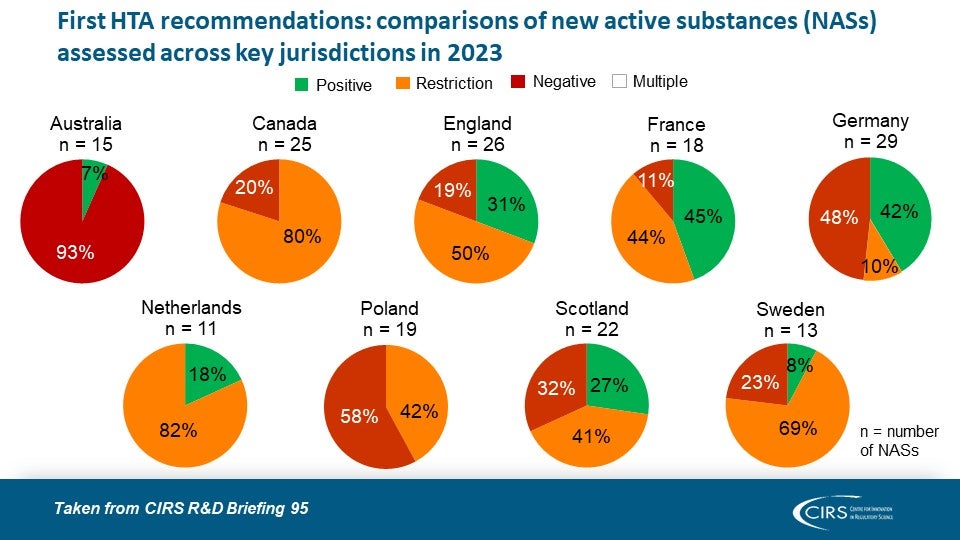
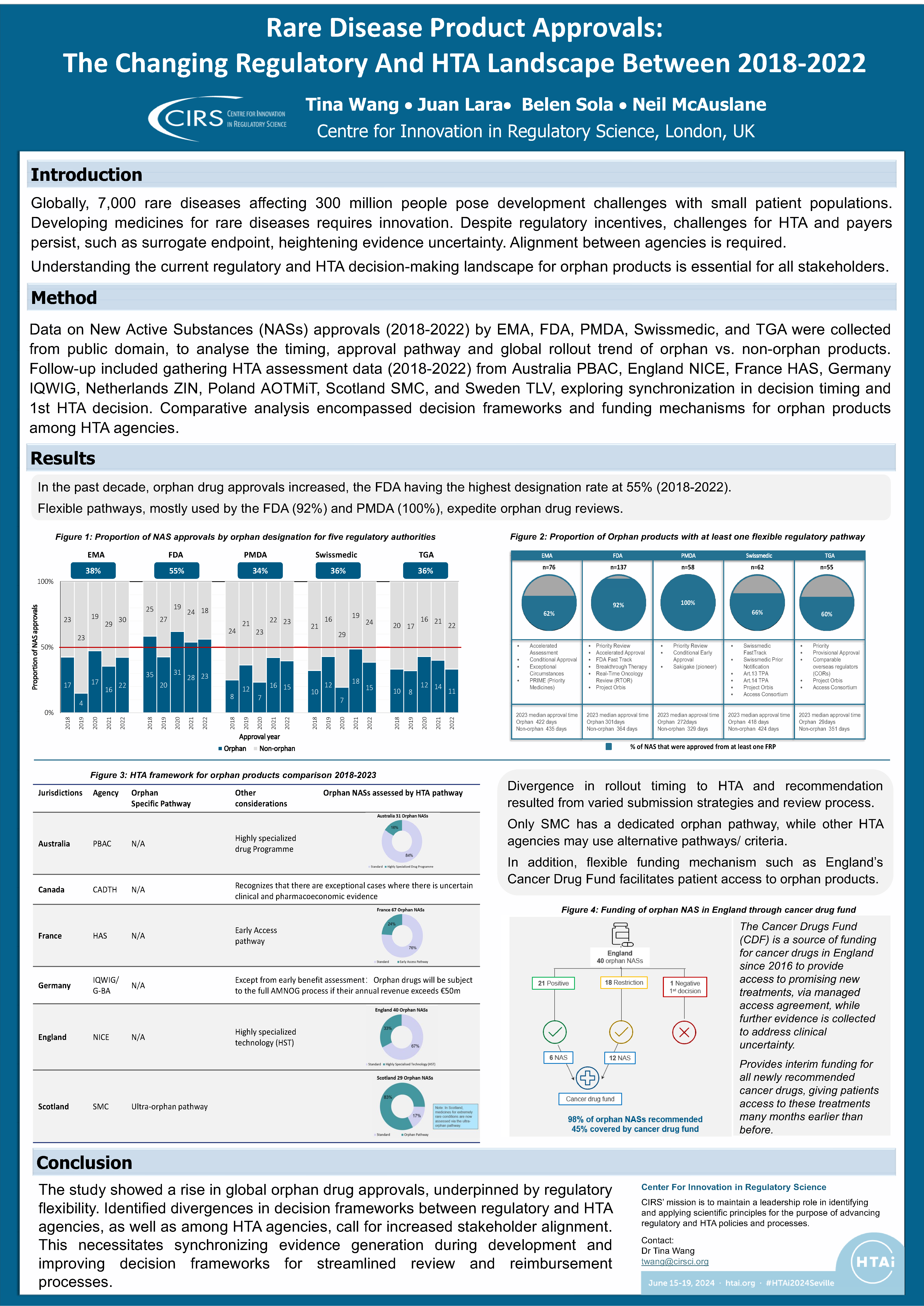
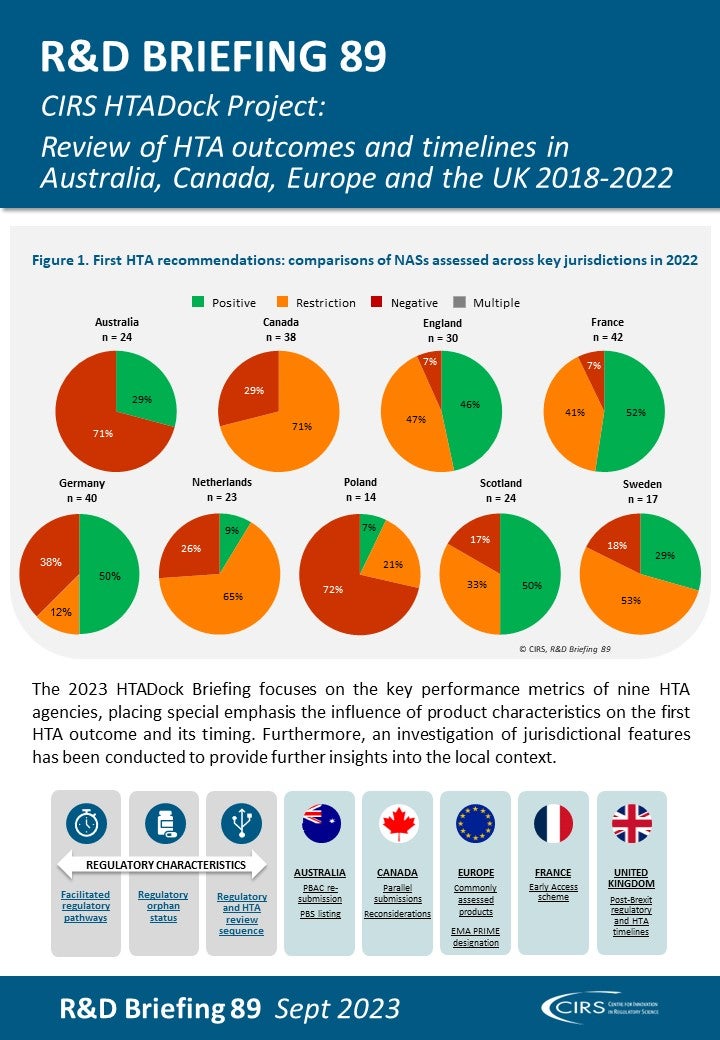
Workshop Report – Regulatory and HTA collaborative models
In this workshop, CIRS brought together senior representatives from regulators, HTA agencies, pharmaceutical companies, payers, academics and patient organisations to discuss the impact of regulatory and HTA collaborative models [...]