Publications
CIRS publishes insights from its research and meetings in several forms:
- R&D Briefings – research papers produced by the CIRS team e.g. annual regulatory and HTA benchmarking briefings
- Journal articles – peer reviewed academic research papers
- Reports – from CIRS workshops and externally commissioned research projects, as well as CIRS Annual Reports
- Books – research theses from CIRS-supported PhD students
- Posters – presented at external conferences
Keep up-to-date with CIRS publications and activities by signing up to our mailing list or following CIRS on LinkedIn.
Testing the UMBRA benefit-risk tool in a South African agency
Objectives Regulators must balance medicine benefits and risks while ensuring consistency, transparency, and efficiency in decision-making and the aim of this study was to assess UMBRA’s impact on SAHPRA to …
Assessing Good Review Practices at the FDA Ghana
Objectives To identify the current perspective of the FDA Ghana in the implementation of Good Review Practices, provide a baseline on the knowledge, attitudes, practices, as well as identify areas …
Proposed Model for EAC MRH Joint Review
Objectives 1. To investigate ways in which the regional initiative could be well coordinated. 2. To propose an improved model for the East African Medicine Regulatory Harmonisation Initiative joint assessments. …
Evaluating Quality of Decision Making within the Zambia Medicines Regulatory Authority
Objectives To assess the quality of the decision-making process followed by regulatory reviewers, explore their perceptions of the Zambia Medicines Regulatory Authority (ZAMRA) decision-making approach, and evaluate the usefulness of …
Regulatory Collaboration and System Strengthening – Workshop Synopsis
This CIRS multi-stakeholder workshop examined success factors for strengthening regulatory systems to support the implementation of collaborative models.
Workshop Report – Regulatory and HTA collaborative models
In this workshop, CIRS brought together senior representatives from regulators, HTA agencies, pharmaceutical companies, payers, academics and patient organisations to discuss the impact of regulatory and HTA collaborative models and …
Ensuring efficiency and effectiveness of Joint Clinical Assessment (JCA) – Wang 2025
Background: This study explored the readiness and strategic considerations of companies and key stakeholders for the implementation of the Joint Clinical Assessment (JCA) under the European Health Technology Assessment Regulation …
CIRS RD Briefing 97 – Access Consortium and Project Orbis Approvals Across Eight Regulators
This R&D Briefing builds upon the Centre for Innovation in Regulatory Science (CIRS)’s long-standing efforts to examine trends and practices in regulatory approvals. For over 20 years, CIRS has been …
Economic impact of reliance on an African regulator – Danks 2025
Background and Objectives The inherited backlog of 16,000 medicines applications of the South African Health Products Regulatory Authority (SAHPRA) was cleared through facilitated review pathways that included reliance on prior …
Review of 2024 CIRS activities
We’re pleased to share a high-level summary of what CIRS got up to last year, including key research outputs and meetings. Our full 2024 Annual Report will be published in …
Suggested Improvements to the EAC-MRH Joint Review Process – Ngum 2025
Background In 2012, the East African Community Medicines Regulatory Harmonization (EAC-MRH) initiative was established to improve access to safe, effective, and high-quality medical products to patients in the East African …
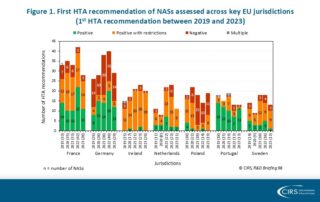
CIRS RD Briefing 98 – European HTA trends: HTA outcomes and timelines across seven markets 2019-2023
This R&D Briefing presents data from HTADock, an ongoing CIRS metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies. It focuses …