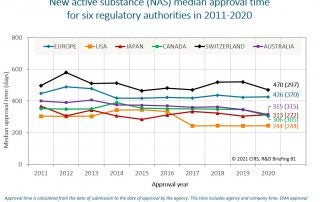
CIRS RD Briefing 81 – New drug approvals in six major authorities 2011-2020
This Briefing presents the results from the CIRS annual analysis of New Active Substance (NAS) approvals by six major regulatory agencies: the European Medicines Agency (EMA), the US Food [...]