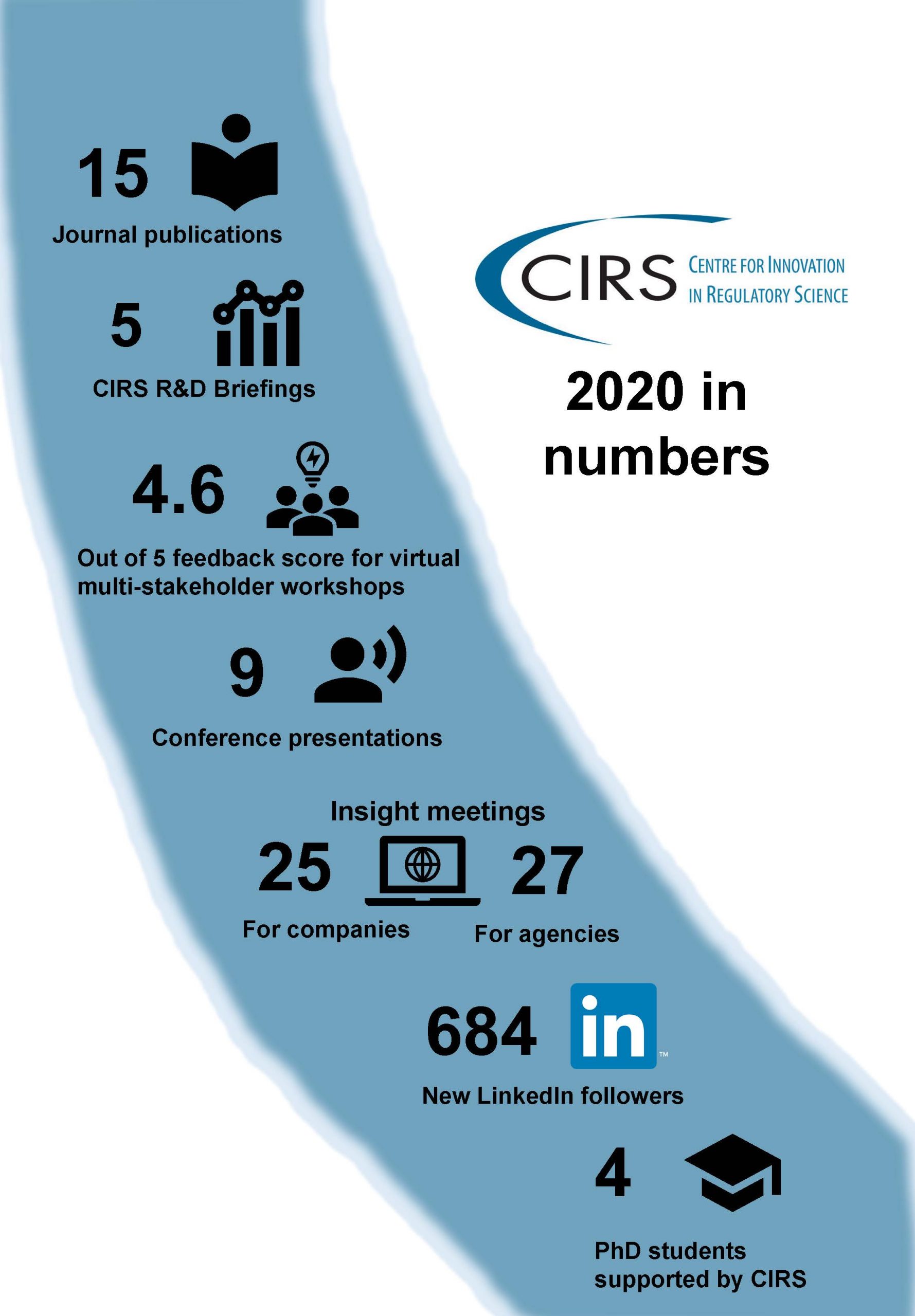
We’re delighted to present our latest Annual Report, which provides a summary of CIRS projects and workshops undertaken in 2020 as well as case studies depicting CIRS’ impact on promoting regulatory reliance and benchmarking HTA agency performance.
CIRS has a rich history of helping to improve the regulatory and access landscape through its work in the areas of metrics, quality and alignment. As regulatory science continues to evolve, we will look to build on these areas but with the background of changes accelerated during the COVID-19 pandemic that facilitate medicines development, review and reimbursement.