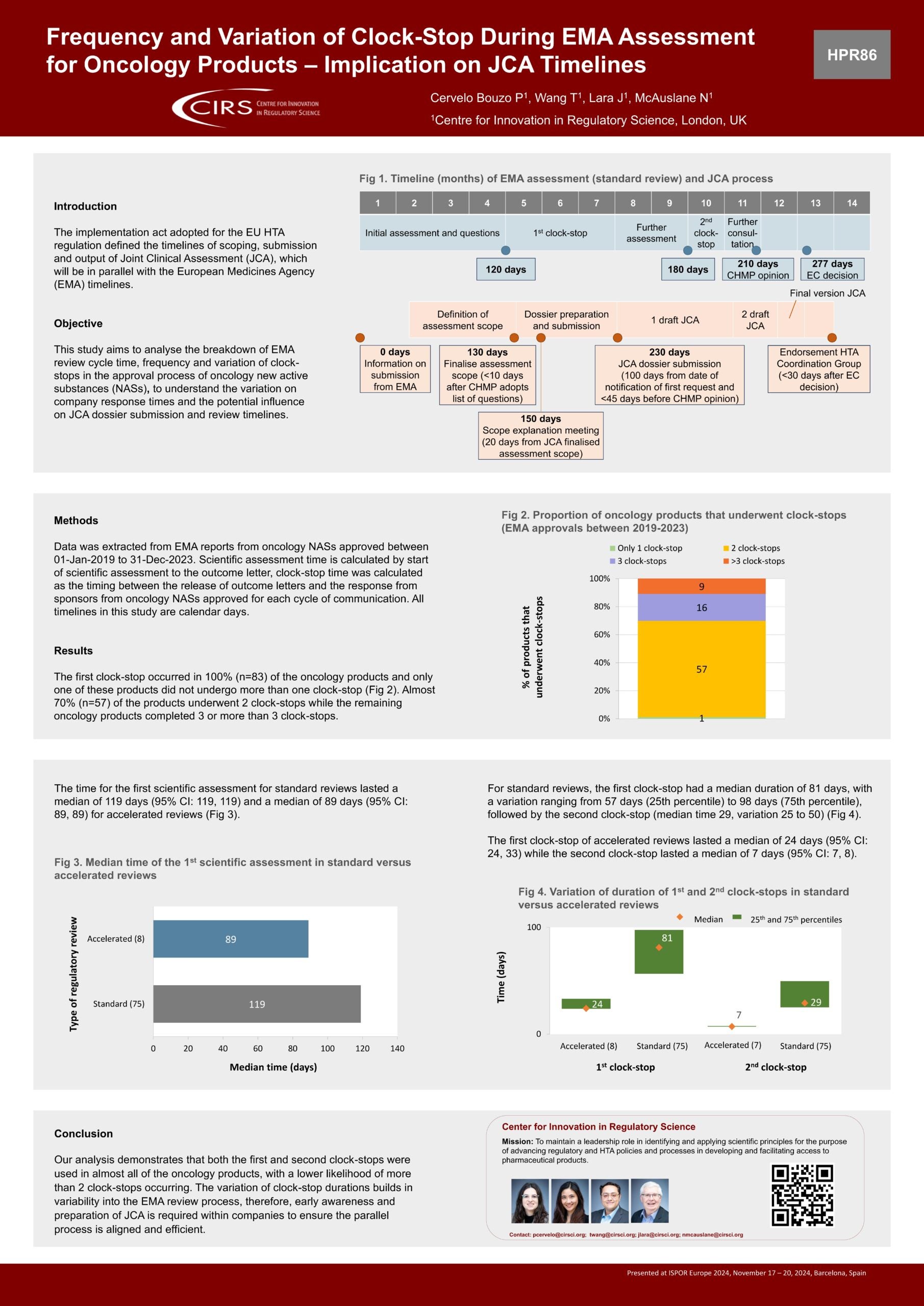
Frequency and Variation of Clock-Stop During EMA Assessment for Oncology Products – Implication on JCA Timelines
Objectives: The implementation act adopted for the HTA Regulation (HTAR) defined the timelines of scoping, submission and assessment and output of Joint Clinical Assessment (JCA), which will be in [...]