CIRS RD Briefing 82 – Regulatory reliance pathways: opportunities and barriers
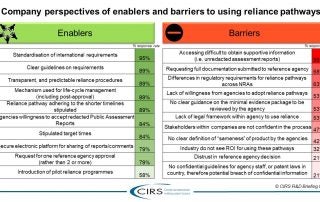
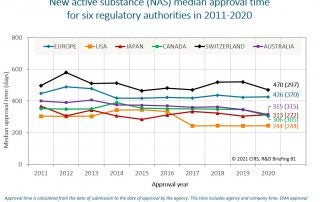
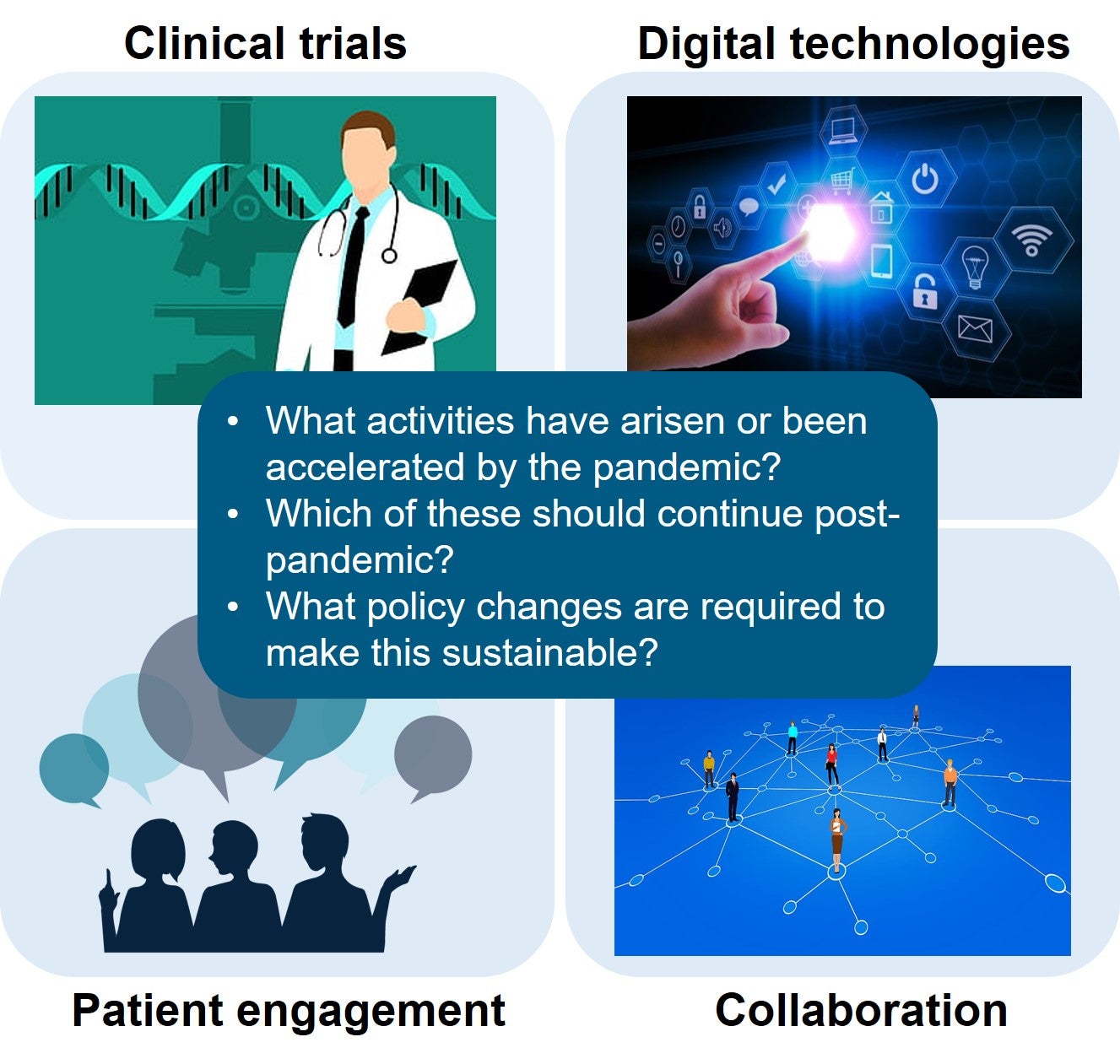
An increasing number of National Regulatory Authorities (NRAs) are turning to reliance as a way to conserve resources, build expertise and capacity, increase the quality of their regulatory decisions, [...]