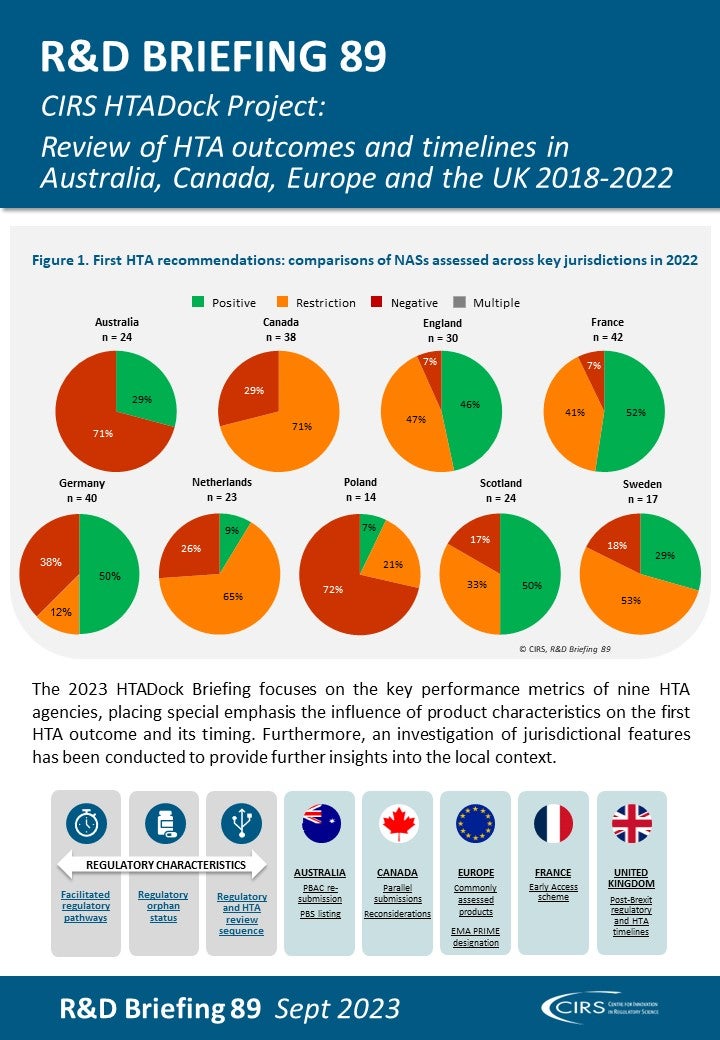
Health technology assessment (HTA)
2023 Workshop Synopsis – Regulatory and reimbursement frameworks for rare disease products
This multi-stakeholder workshop consisted of a series of presentation sessions and three parallel breakout discussions. Presentations explored trends in regulatory and HTA approvals of orphan products [...]