Publications
CIRS publishes insights from its research and meetings in several forms:
- R&D Briefings – research papers produced by the CIRS team e.g. annual regulatory and HTA benchmarking briefings
- Journal articles – peer reviewed academic research papers
- Reports – from CIRS workshops and externally commissioned research projects, as well as CIRS Annual Reports
- Books – research theses from CIRS-supported PhD students
- Posters – presented at external conferences
Keep up-to-date with CIRS publications and activities by signing up to our mailing list or following CIRS on LinkedIn.
Keyter et al 2021 – Impact of reliance on South African review
Background: The aims of this study were to compare the overall regulatory review timelines achieved by the South African Health Products Regulatory Authority (SAHPRA) in 2020 to the timelines historically achieved …
2020 Workshop report – Effectiveness of the regulatory approval process
This workshop was part of a series of global development workshops that brought together mature and maturing regulatory agencies. These workshops successively built on one another and evolved from focusing …
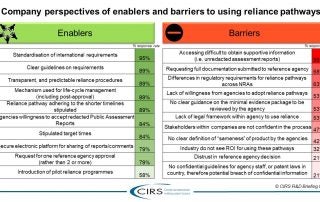
CIRS RD Briefing 82 – Regulatory reliance pathways: opportunities and barriers
An increasing number of National Regulatory Authorities (NRAs) are turning to reliance as a way to conserve resources, build expertise and capacity, increase the quality of their regulatory decisions, reduce …
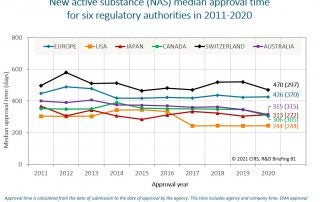
CIRS RD Briefing 81 – New drug approvals in six major authorities 2011-2020
This Briefing presents the results from the CIRS annual analysis of New Active Substance (NAS) approvals by six major regulatory agencies: the European Medicines Agency (EMA), the US Food and …
CIRS 2020 Annual Report
We’re delighted to present our latest Annual Report, which provides a summary of CIRS projects and workshops undertaken in 2020 as well as case studies depicting CIRS’ impact on promoting …
Bujar et al 2021 – Transparency in EMA and FDA decision making
Although it cannot be expected that different medicines’ regulatory agencies always reach the same review outcome, it is important that decision making is documented and communicated to ensure transparency. This …
CIRS RD Briefing 80 – Reimagining medicine regulatory models
This R&D Briefing summarises the outputs of breakout group discussions held during a CIRS multi-stakeholder workshop in December 2020 entitled ‘Reimagining medicine regulatory models: implementing fit-for-purpose sustainable activities for patient …
CIRS RD Briefing 79 – Use of advisory committees in Colombia
This Briefing provides an overview of how advisory committees can be used to support the regulatory decision-making process and considering the context of Latin American regulatory systems, aims to better …
Bujar et al 2021 – Value of Facilitated Regulatory Pathways
Background: Despite the growing application of facilitated regulatory pathways (FRPs), little attention has focused on assessing the perception of pharmaceutical companies regarding their usefulness beyond increasing timeliness. Objectives: The aim …
Sithole et al 2021 – Regulatory review process in Zimbabwe
Purpose: The aims of this study were to assess the current regulatory review process of the Medicines Control Authority of Zimbabwe (MCAZ), identify key milestones and target timelines, evaluate the …
Wang et al 2020 – Companies’ HTA strategies and practices
Background: Health technology assessment (HTA) has increased in importance in supporting payer decision making by assessing the relative effectiveness and cost effectiveness of new medicines. Thus, pharmaceutical companies need to address …