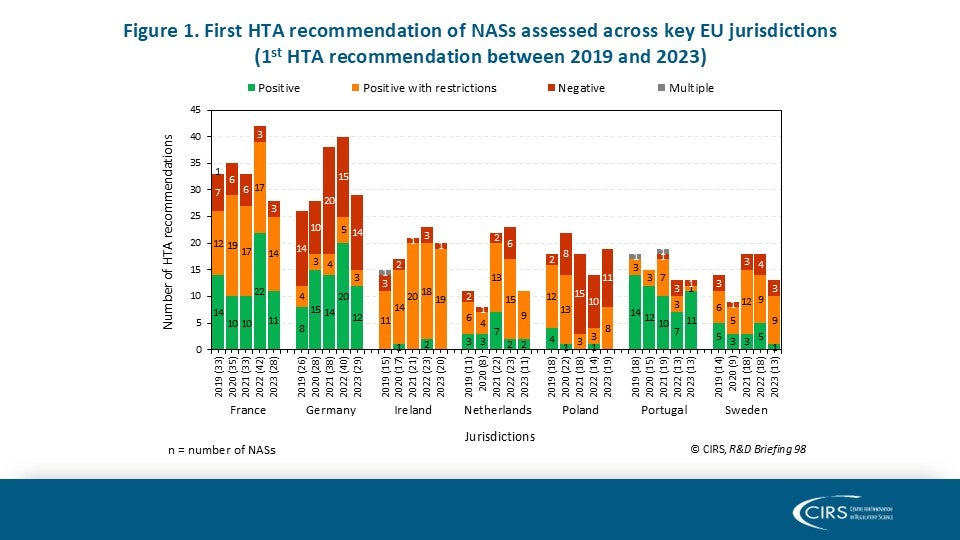
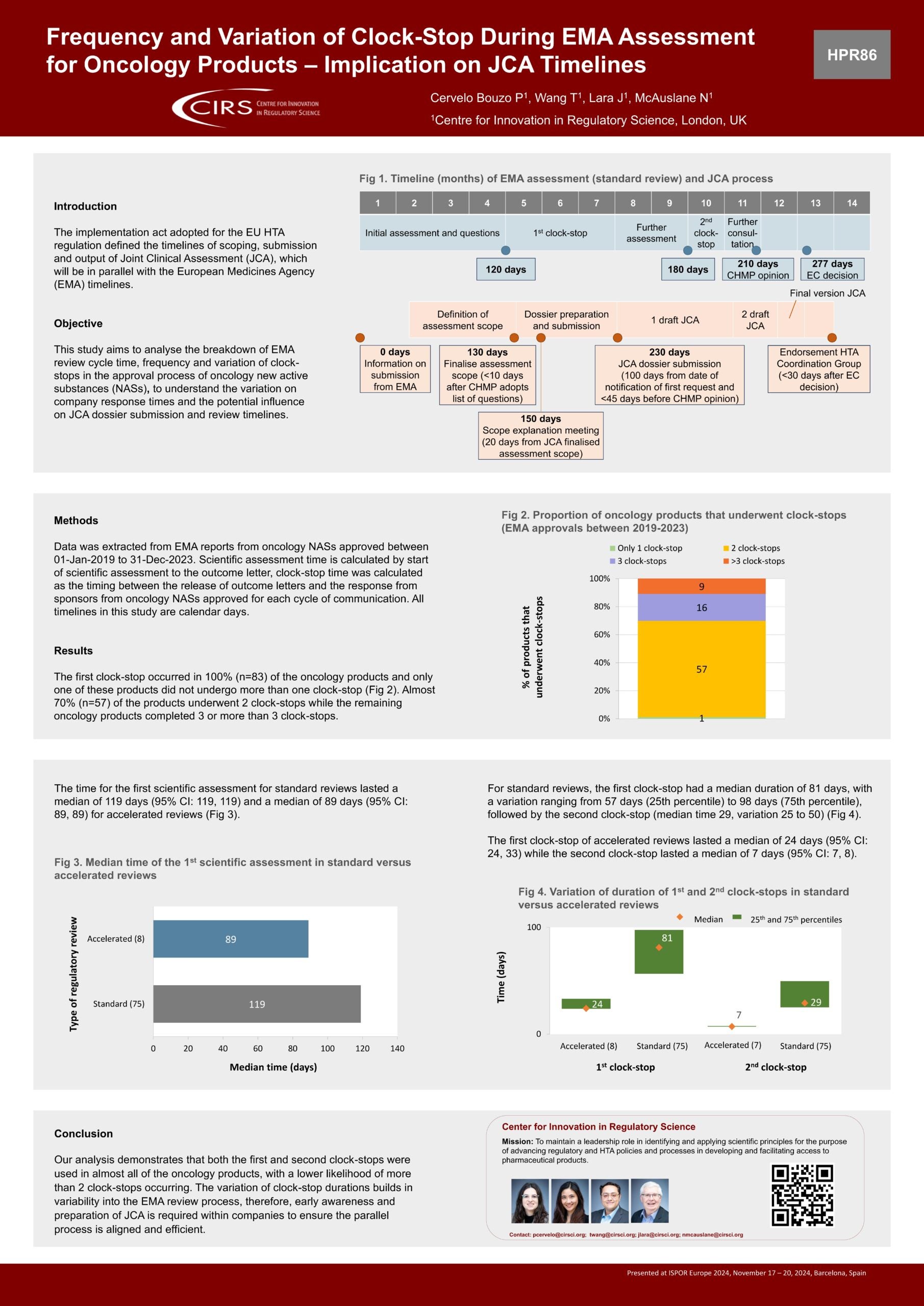
Suggested Improvements to the EAC-MRH Joint Review Process – Ngum 2025
Background In 2012, the East African Community Medicines Regulatory Harmonization (EAC-MRH) initiative was established to improve access to safe, effective, and high-quality medical products to patients in the East [...]