Publications
CIRS publishes insights from its research and meetings in several forms:
- R&D Briefings – research papers produced by the CIRS team e.g. annual regulatory and HTA benchmarking briefings
- Journal articles – peer reviewed academic research papers
- Reports – from CIRS workshops and externally commissioned research projects, as well as CIRS Annual Reports
- Books – research theses from CIRS-supported PhD students
- Posters – presented at external conferences
Keep up-to-date with CIRS publications and activities by signing up to our mailing list or following CIRS on LinkedIn.
CIRS 2021 Annual Report
We’re delighted to present our latest Annual Report, which provides a summary of CIRS projects, publications and workshops undertaken last year as well as case studies depicting CIRS’ impact in …
2021 Workshop report – Digital technologies for clinical evidence generation
Digitisation and digital health technologies are transforming clinical development; companies, regulators and Health Technology Assessment (HTA) agencies are looking to derive actionable insights from the data being generated. This is …
2021 Workshop report – Regulatory, HTA and payer interactions and collaborations
Over the last five years, regulatory and HTA interactions, as well multi-HTA and multi-regulatory interactions and collaborations, have evolved in thinking and mutual activities both at a product level as …
2020 Workshop report – Reimagining regulatory models
As the regulatory landscape changes to meet new challenges, such as increasingly sophisticated medical innovations, fundamental questions are being raised: What is the role of a ‘modern’ regulator today? Does …
Comparison of the registration process of Zimbabwe with Australia, Canada, Singapore and Switzerland
Background: Benchmarking regulatory systems of low- and middle-income countries with mature systems provides an opportunity to identify gaps, enhance review quality, and reduce registration timelines, thereby improving patients’ access to …
Seeking early scientific advice from HTA agencies
There is a growing trend for pharmaceutical companies to seek scientific advice on drug development from a Health Technology Assessment (HTA) perspective, to improve the efficiency of their studies, enable …
Regulatory-HTA decision-making interface: what the medical writer should know
For a new medicine to reach patients, it must achieve both regulatory marketing authorisation and reimbursement from the payer. Because regulators assess the benefits and risks of a medicine while …
Review models and timelines in the Southern African Development Community
Introduction: Regulatory reliance, harmonization and work sharing have grown over the last few years, resulting in greater sharing of work and information among regulators, enabling efficient use of limited resources …
Good Review Practices in the Southern African Development Community
Introduction: National medicines regulatory agencies are faced with challenges including limited resources and technical capacity, resulting in countries collaborating and sharing resources to improve efficiency of the review process to facilitate …
2021 Project report – Monitoring implementation and adherence to ICH guidelines
Background: This study was built on the previous 2018/2019 assessment where ICH selected the Centre for Innovation in Regulatory Science (CIRS) to collaborate on the development and the conduct of …
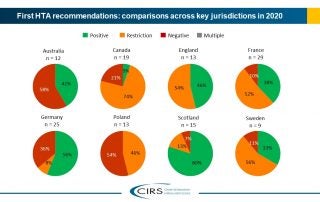
CIRS RD Briefing 83 – HTA outcomes in Australia, Canada and Europe 2016-2020
This Briefing presents data from HTADock, an ongoing metrics study that collects data on new active substances (NASs) appraised by eight HTA agencies and analyses synchronisation between the regulatory decision …