Publications
CIRS publishes insights from its research and meetings in several forms:
- R&D Briefings – research papers produced by the CIRS team e.g. annual regulatory and HTA benchmarking briefings
- Journal articles – peer reviewed academic research papers
- Reports – from CIRS workshops and externally commissioned research projects, as well as CIRS Annual Reports
- Books – research theses from CIRS-supported PhD students
- Posters – presented at external conferences
Keep up-to-date with CIRS publications and activities by signing up to our mailing list or following CIRS on LinkedIn.
Evolution of HTA practice and approaches from the perspectives of HTA agencies and pharmaceutical industry
Health technology assessment (HTA) has emerged as an important tool to support healthcare decision-makers to make rational reimbursement decisions, with the ultimate purpose of promoting an efficient healthcare system. HTA …
2021 Workshop report – Regulatory and HTA landscape in Asia and Latin America
As regulatory systems mature with demand for new innovative treatments, jurisdictions are seeking to introduce more comprehensive healthcare systems; this is often accompanied by efforts to initiate health technology assessment …
Assessment of the effectiveness and efficiency of the West Africa medicines regulatory harmonization initiative by the member countries
Background: The West Africa Health Organization launched the West Africa Medicines Regulatory Harmonization Project (WA-MRH) in 2017 with the overarching objective to improve the availability of high-quality, safe and effective …
Evaluation of the Food and Drugs Authority, Ghana Regulatory Review Process: Challenges and Opportunities
Purpose: This study aimed to assess the current regulatory review process of the food and drugs authority (FDA) Ghana by identifying key milestones, target timelines, good review practices and quality …
Evaluation of the East African Community joint assessment procedure by pharmaceutical companies
Background: A 2021 study to determine the viewpoints among the seven member countries regarding the effectiveness (i.e., achieving the intended outcomes) and efficiency (i.e., achieving the intended outcomes in timely …
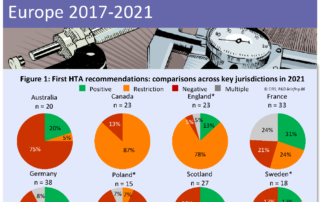
CIRS RD Briefing 86 – Review of HTA outcomes and timelines in Australia, Canada and Europe 2017-2021
The Briefing presents data from HTADock, an ongoing metrics study that collects data on new active substances (NASs) appraised by eight HTA agencies and analyses synchronisation between the regulatory decision …
Regulatory work-sharing initiative in Africa: ZaZiBoNa, past, present and future
PREFACE: The role of regulatory authorities in the health system is to ensure the quality, safety and efficacy of medical products. It is acknowledged that regulatory authorities are at times …
Evaluation of the East African Community joint assessment
Background: For almost a decade, the East African Community has implemented the Medicines Regulatory Harmonization (EAC-MRH) programme among its member states to harmonise technical requirements and standards for medical products …
Building HTA/Payer Perspectives Into Drug Development
Background: The target product profile (TPP) outlines the desired profile of a target product aimed at a particular disease and is used by companies to plan clinical development. Considering the increasing …
Evaluation of the performance of the Gulf centralised registration procedure
Background: The Gulf Centralised Committee for Drug Registration (GCC-DR), as part of the Gulf Health Council (GHC), enables the consolidated registration of pharmaceutical products throughout the member states of the …
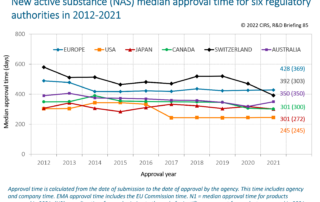
CIRS RD Briefing 85 – New drug approvals in six major authorities 2012-2021
This Briefing presents the results from the CIRS annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the European Medicines Agency (EMA), the US Food and …
Industry evaluation of the efficiency and effectiveness of ZaZiBoNa
Introduction: The common technical document (CTD) format harmonised the requirements for the registration of medicines, which had traditionally differed from country to country, making it possible for countries to collaborate …
Regulatory evaluation of the efficiency and effectiveness of ZaZiBoNa
Introduction: ZaZiBoNa, the work-sharing initiative in the Southern African Development Community (SADC) that has been in operation for 8 years has successfully assessed over 300 dossiers/applications, with an overall median …
CIRS RD Briefing 84 – China’s evolving regulatory landscape
China has made significant changes to its medicine regulatory system including: Regulatory reforms – since 2015, regulatory reforms have helped to eliminate application backlogs, improve review timelines and increase approvals …