Publications
CIRS publishes insights from its research and meetings in several forms:
- R&D Briefings – research papers produced by the CIRS team e.g. annual regulatory and HTA benchmarking briefings
- Journal articles – peer reviewed academic research papers
- Reports – from CIRS workshops and externally commissioned research projects, as well as CIRS Annual Reports
- Books – research theses from CIRS-supported PhD students
- Posters – presented at external conferences
Keep up-to-date with CIRS publications and activities by signing up to our mailing list or following CIRS on LinkedIn.
Evaluation of the Effectiveness and Efficiency of Ten Years’ Experience with the East Africa Community Joint Assessment
During the DIA Global Annual Meeting 2023, Nancy Yang-Ngum developed and presented a poster in which she shared the results, recommendations and conclusions about the topic “Evaluation of the Effectiveness …
Evaluation of the Regulatory Review Process of the FDA Ghana: Challenges and Opportunities for Improvement
During the DIA Global Annual Meeting 2023, Mercy Owusu-Asante developed and presented a poster in which she shared the results, recommendations and conclusions about the topic “Evaluation of the Regulatory …
Evaluation of the impact of reliance on the regulatory performance in the South African Health Products Regulatory Authority
During the DIA Global Annual Meeting 2023, Lorraine Danks, Boitumelo Semete-Makokotlela, Sam Salek and Stuart Walker developed and presented a poster in which they shared the results, recommendations and conclusions …
A comparison of the Regional Medicines Regulatory Harmonisation Projects in East, West and Southern Africa.
During the DIA Global Annual Meeting 2023, Tariro Sithole, Nancy Ngum, Mercy Owusu-Asante, Stuart Walker and Sam Salek developed and presented a poster in which they shared the results, recommendations …
A comparison of regulatory decision patterns for oncology products to all other non-oncology products among Swissmedic, EMA and FDA
Consensus of regulatory decisions on the same Marketing Authorization Application (MAA) are critical for stakeholders. In this context, regulatory decision patterns from the Swissmedic (SMC), the US Food and Drug …
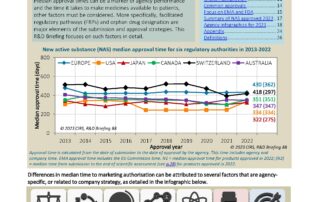
CIRS RD Briefing 88 – New drug approvals in six major authorities 2013-2022: Focus on orphan designation and facilitated regulatory pathways
This R&D Briefing presents the results from the Centre for Innovation in Regulatory Science (CIRS) annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the European …
CIRS RD Briefing 87 – A Roadmap for Regulatory Strengthening: CIRS Tools for Measuring and Optimising Regulatory Performance to Support Practices in Line with the World Health Organization Global Benchmarking Tool Indicators
Over the last 20 years, CIRS has been developing regulatory science tools to increase transparency of processes, support quality regulatory decision making, and provide global advocacy in support of regulatory …
Evaluation of Risk‑Based Approaches to the Registration of Medicines: Current Status Among African Regulatory Authorities
Background: Despite the worldwide need for increased access to safe and effective medicines, there is a lack of innovative medicines in many low- to middle-income countries. On the African continent, …
Regulatory performance of the East African Community joint assessment procedure: The way forward for regulatory systems strengthening
Background: Seven national medicines regulatory authorities in the East African Community (EAC) have embraced regulatory reliance, harmonization and work sharing through the EAC Medicines Regulatory Harmonization programme. Measuring the performance …
Assessment of the effectiveness and efficiency of the West Africa medicines regulatory harmonization initiative by the pharma industry
Background: Following the establishment of Economic Community of West African States Medicines Regulatory Harmonization (ECOWAS-MRH) initiative in 2017, it was considered timely to carry out an evaluation of the current status …
2022 Workshop report – Building on regulatory and HTA agilities for high unmet need
In 2020, coronavirus disease (COVID-19) spread rapidly around the world and halted regular social, business, and research activities. The pandemic impeded normal functioning across several fields, and businesses and agencies …
2022 Workshop report – Collaborative models for regionalisation, work and information sharing: How do these fit into the regulatory toolkit?
This workshop looked at how maturing markets are building risk-based approaches into regulatory assessment, building on recent CIRS workshops in Singapore (2019) and South Africa (2018). The workshop also explored …
Regulatory, HTA and company interactions: the current landscape and future ecosystem for drug development, review and reimbursement
Background: Multi-stakeholder interactions have evolved at product and policy levels. There is a need to assess the current and future landscape of interactions between companies, and regulatory and HTA agencies to …
CIRS 2022 Annual Report
We’re delighted to present our latest Annual Report, which provides a summary of the projects, publications and Multi-stakeholder Workshops, Technical Fora, Industry Discussion Meetings, and Impact Case Studies from Regulatory …
2022 Workshop report – How has the pandemic accelerated the acceptance and utility of RWD/RWE in regulatory/HTA decision making?
This workshop builds on the outcomes of the CIRS Professor Breckenridge memorial workshop in December 2020, as well as the 2021 workshop on utilisation of digital technologies in clinical development. …