Publications
CIRS publishes insights from its research and meetings in several forms:
- R&D Briefings – research papers produced by the CIRS team e.g. annual regulatory and HTA benchmarking briefings
- Journal articles – peer reviewed academic research papers
- Reports – from CIRS workshops and externally commissioned research projects, as well as CIRS Annual Reports
- Books – research theses from CIRS-supported PhD students
- Posters – presented at external conferences
Keep up-to-date with CIRS publications and activities by signing up to our mailing list or following CIRS on LinkedIn.
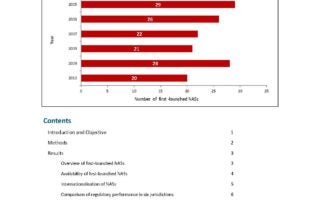
CIRS RD Briefing 55 – Approvals across six major authorities 2004-2013
As part of the ongoing study to monitor regulatory performance, CIRS has analysed the trends in new medicines’ approval between 2004 and 2013 by six regulatory authorities including Health Canada, …
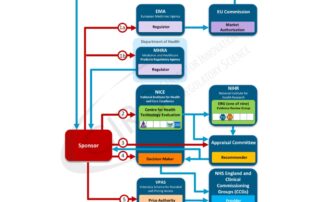
2013 Workshop report – Commonality across decision frameworks used by HTA and regulatory agencies
IS THERE A COMMONALITY ACROSS THE STRUCTURED DECISION FRAMEWORKS USED BY HTA AND REGULATORY AGENCIES? 1st-2nd October 2013, Surrey, UK This Workshop was designed to bring together the various stakeholders …
CIRS RD Briefing 54 – Approvals in ICH countries 2004-2013
In 2013, the overall number of New Active Substances (NASs) approved by EMA, FDA and PMDA was comparable across the three agencies. Nevertheless, despite this similarity, the number of NASs …
CIRS RD Briefing 53 – Factors influencing drug roll out to six mature markets
Objective: To review NASs first launched between 2005‐2010 and to determine their regulatory status as of 31 December 2012 in USA, Europe, Japan, Canada, Switzerland and Australia to identify the …
Liu et al 2013 – Characterising Good Review Practices across APEC agencies
As a first step in the implementation of the Asia-Pacific Economic Cooperation (APEC) Best Regulatory Practice Project, the Centre for Innovation in Regulatory Science conducted a gap analysis survey among …
Allen et al 2013 – Archetypes for non-ranking classification and comparison of European HTA systems
Introduction: European countries are increasingly utilising health technology assessment (HTA) to inform reimbursement decision-making. However, the current European HTA environment is very diverse, and projects are already underway to initiate …
CIRS RD Briefing 52 – New drug approvals in ICH countries 2003-2012
Active Substances (NASs) approved by both the FDA and PMDA represented the largest number of new medicines approved this decade. Regulatory approvals by EMA were lower than the other agencies …
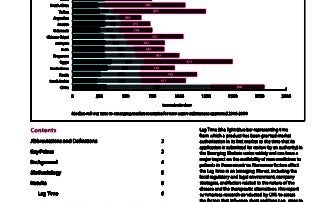
CIRS RD Briefing 51 – Submission lag time in the emerging markets
Lag Time (the time from which a product has been granted market authorisation in its first market to the time that its application is submitted for review by an authority) …
2011 Workshop report – Evolving the regulatory review process
Evolving the regulatory review process: What are the features that enable a transparent, timely, predictable and good quality review? 6-7th December 2011, Kuala Lumpur, Malaysia This workshop was held to bring …
CIRS RD Briefing 51 – New drug approvals in ICH countries 2002-2011
In 2011, the number of New Active Substances (NASs) approved in all the ICH countries increased compared to 2010, and FDA represented the largest number of new medicines approved this …
Salek et al 2012 – Scorecards to Assess the Quality of a Regulatory Submission and Its Review
An efficient review depends not only on timely approval but also on ensuring the quality of the process from construction of the dossier to the ultimate regulatory decision. Two scorecards …
Liberti et al 2010 – Progress on the development of a benefit-risk framework for evaluating medicines
CIRS authors write about benefit-risk in the March 2010 issue of Regulatory Focus by the Regulatory Affairs Professionals Society (RAPS). Article reproduced with the permission of RAPS. Download
Hirako et al 2007 – Comparison of the drug review process at five international regulatory agencies
Regulatory approval time is a key metric that is used to evaluate the performance of regulatory agencies. A new methodology has been developed to compare the regulatory review process across …
CIRS RD Briefing 46: Building quality into regulatory activities
The Institute for Regulatory Science is currently involved in several activities related to quality as it applies to regulatory submissions and procedures, rather than the more conventional association with the …
CIRS RD Briefing 50 – Cross-regional comparison of regulatory environment in emerging markets
The emerging markets of Asia-Pacific, the Middle East, Africa and Latin America are becoming increasingly important to pharmaceutical companies in their global strategies for the registration of new medicines and …