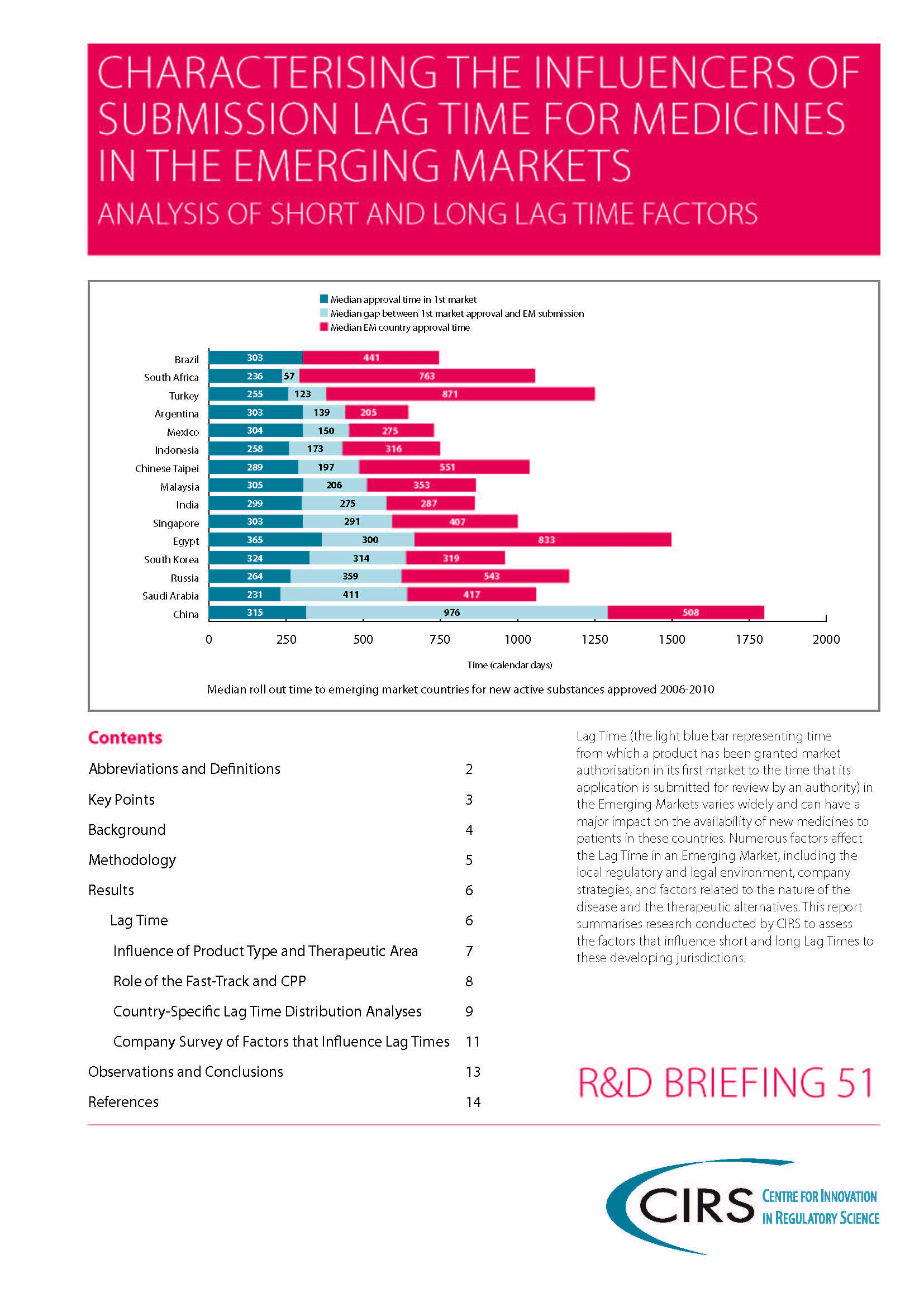
Lag Time (the time from which a product has been granted market authorisation in its first market to the time that its
application is submitted for review by an authority) in the Emerging Markets varies widely and can have a major impact on the availability of new medicines to patients in these countries. Numerous factors affect the Lag Time in an Emerging Market, including the local regulatory and legal environment, company strategies, and factors related to the nature of the disease and the therapeutic alternatives. This report summarises research conducted by CIRS to assess the factors that influence short and long Lag Times to these developing jurisdictions.