Publications
CIRS publishes insights from its research and meetings in several forms:
- R&D Briefings – research papers produced by the CIRS team e.g. annual regulatory and HTA benchmarking briefings
- Journal articles – peer reviewed academic research papers
- Reports – from CIRS workshops and externally commissioned research projects, as well as CIRS Annual Reports
- Books – research theses from CIRS-supported PhD students
- Posters – presented at external conferences
Keep up-to-date with CIRS publications and activities by signing up to our mailing list or following CIRS on LinkedIn.
CIRS RD Briefing 61: Building quality into decision-making processes
In 2015, CIRS initiated a programme in Quality Decision Making with the following aims: Evaluate the current decision-frameworks and understand the characteristics of different decision-making processes Assess the quality of …
Haqaish et al 2017 – Jordan Food and Drug Administration: Comparison of its Registration Process with Australia, Canada, Saudi Arabia and Singapore
Objective: This study compares the current regulatory review process and good review practices at the Saudi Food and Drug Authority (SFDA) with those of regulatory agencies in Australia, Canada, and …
2016 Workshop report – Commonality in evidentiary requirements
Commonality in evidentiary requirements across regulatory and HTA stakeholders 21-22 SEPTEMBER 2016, SURREY, UK Workshop objectives Discuss the progress made to align evidentiary requirements, what the drivers have been and …
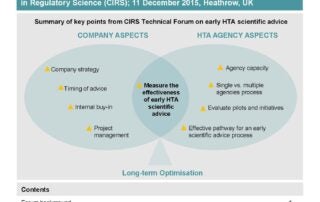
CIRS RD Briefing 60 – Early scientific advice from HTA agencies
This R&D Briefing 60 summarises highlights from the Technical Forum convened by CIRS on 11 December 2015, Heathrow, UK. Forum objectives: Identify companies’ current approaches to seeking early scientific advice …
2016 Workshop report – RWD to RWE for assessing efficacy and effectiveness
Real-world data to real-world evidence for assessing efficacy and effectiveness: Opportunities and challenges for new medicines development, regulatory review and health technology assessment 23-24 JUNE 2016 TYSONS CORNER, VIRGINIA, US …
CIRS RD Briefing 59 – New approvals in six regulatory authorities 2006-2015
The last decade, 2006-2015, has seen a continuation of the convergence and general decrease in the approval times amongst six major regulatory authorities, namely the European Medicines Agency (EMA), the …
2016 Workshop report – Key performance metrics for regulatory processes
WHAT ARE THE KEY PERFORMANCE METRICS THAT AGENCIES AND COMPANIES SHOULD USE TO MEASURE REGULATORY PROCESSES AND PRACTICES TO FACILITATE THE LICENSING OF NEW MEDICINES? 3-4 FEBRUARY 2016, KUALA LUMPUR, …
Hashan et al 2016 – Saudi Arabia FDA: An Evaluation of the Registration Process and Good Review Practices in Saudi Arabia in Comparison with Australia, Canada and Singapore
Objective: This study compares the current regulatory review process and good review practices at the Saudi Food and Drug Authority (SFDA) with those of regulatory agencies in Australia, Canada, and …
Al-Rubaie et al 2015 – Agency and industry views of the efficiency of the Gulf centralised registration procedure
The aim of this study was to examine the views and experiences of the Gulf Cooperation Council (GCC) states and pharmaceutical companies to identify the strengths and weaknesses of the …
CIRS RD Briefing 58: Changing regulatory environment in Latin America
The aim of this Briefing is to review and summarise the findings from the major studies and interactions carried out by CIRS in LATAM in the last decade in order …
2015 Workshop report – Patient involvement in review and reimbursement
What is the patient’s role in informing the decision process for approval and reimbursement of new medicines? 7-th October 2015, Windsor, UK This workshop built on CIRS workshops on this …
CIRS RD Briefing 57 – New drug approvals in ICH countries 2005-2014
There have been major improvements in the regulatory environment in the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) countries over the last …
CIRS RD Briefing 56: Understanding the dynamics of China’s regulatory environment
Changes have occurred in the organisation and procedural activities of the China Food and Drug Administration (CFDA) and the Centre for Drug Evaluation (CDE). Initiatives that were designed to improve …
Walker et al 2015 – Universal Framework for the Benefit-Risk Assessment of Medicines
A universal framework for the evaluation of the benefit-risk assessment of medicines during development by pharmaceutical companies and in the regulatory review by regulatory authorities is considered of value, as …
Al-Rubaie et al 2014 – Evaluation of the Gulf centralised registration procedure
The aim of the study was to evaluate the Gulf Cooperation Council (GCC) centralized regulatory review process. Regulatory review times—including submission and application dates for new active substances (NASs) and …