Publications
CIRS publishes insights from its research and meetings in several forms:
- R&D Briefings – research papers produced by the CIRS team e.g. annual regulatory and HTA benchmarking briefings
- Journal articles – peer reviewed academic research papers
- Reports – from CIRS workshops and externally commissioned research projects, as well as CIRS Annual Reports
- Books – research theses from CIRS-supported PhD students
- Posters – presented at external conferences
Keep up-to-date with CIRS publications and activities by signing up to our mailing list or following CIRS on LinkedIn.
CIRS RD Briefing 76 – Mexican therapeutic landscape
An efficient regulatory process can be reflected in measurable positive health impacts; conversely, activities that slow or impede regulatory efficiency and predictability can be detrimental. Recent developments in the Mexican …
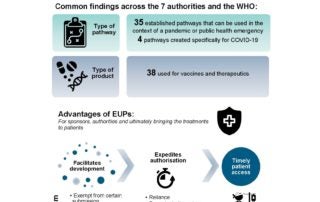
CIRS RD Briefing 75 – Emergency Use Pathways (EUPs)
It has become clear that agencies have a number of pathways that can be used during public health emergencies for the authorisation of therapeutics and vaccines. Some of these so-called …
Keyter et al 2020 – Implementation of a Framework for an Abridged Review Using Good Reliance Practices: Optimising the Medicine Regulatory Review Process in South Africa
Background This study sought to identify criteria and current practices for implementing an abridged review process and understanding barriers and enablers in utilizing reliance models and to offer recommendations for …
Mashaki Ceyhan et al 2020 – Patients’ perspectives of the pharmaceutical regulatory and reimbursement system in Istanbul, Turkey
The aim of this study was to explore patients’ knowledge and perspectives in Istanbul, Turkey about the pharmaceutical regulatory review and reimbursement processes with respect to access to new medicines. …
Koyuncu et al 2020 – Evaluation of the performance of the Turkish regulatory agency: recommendations for improved patients’ access to medicines
Background: This study was to evaluate the Turkish regulatory review process and timelines between 2016 and 2018 with a view to assess the changes that had taken place since the …
CIRS RD Briefing 74 – OpERA programme
CIRS has collected regulatory assessment data for over 20 years, initially with ICH and ICH-observing countries. The OpERA programme, “Optimising Efficiencies in Regulatory Agencies (OpERA)”, was initiated through CIRS in …
Keyter et al 2019 – Evaluation of the performance of the South African regulatory agency
Background: Timely access to new medicines may be addressed through strengthening of registration efciencies and timelines by establishing and refning value-added registration processes, resources, and systems. The aims of this …
Kühler et al 2019 – To what degree are review outcomes aligned for new active substances between the EMA and the US FDA?
Objective To compare review outcome alignment between European Medicines Agency (EMA) and US Food and Drug Administration (FDA) for medicines approved by both agencies in the time period 2014–2016. Design …
Project report – Monitoring implementation and adherence to ICH guidelines
Background: According to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Articles of Association, the regulatory members of ICH are expected to implement ICH …
Executive Colloquium report – What is the value and return on investment for our company to maintain a regulatory policy function?
In June 2019, the Centre for Innovation in Regulatory Science (CIRS) held an Executive Colloquium in Rockville, MD, USA that brought together representatives from multinational pharmaceutical companies to gauge their …