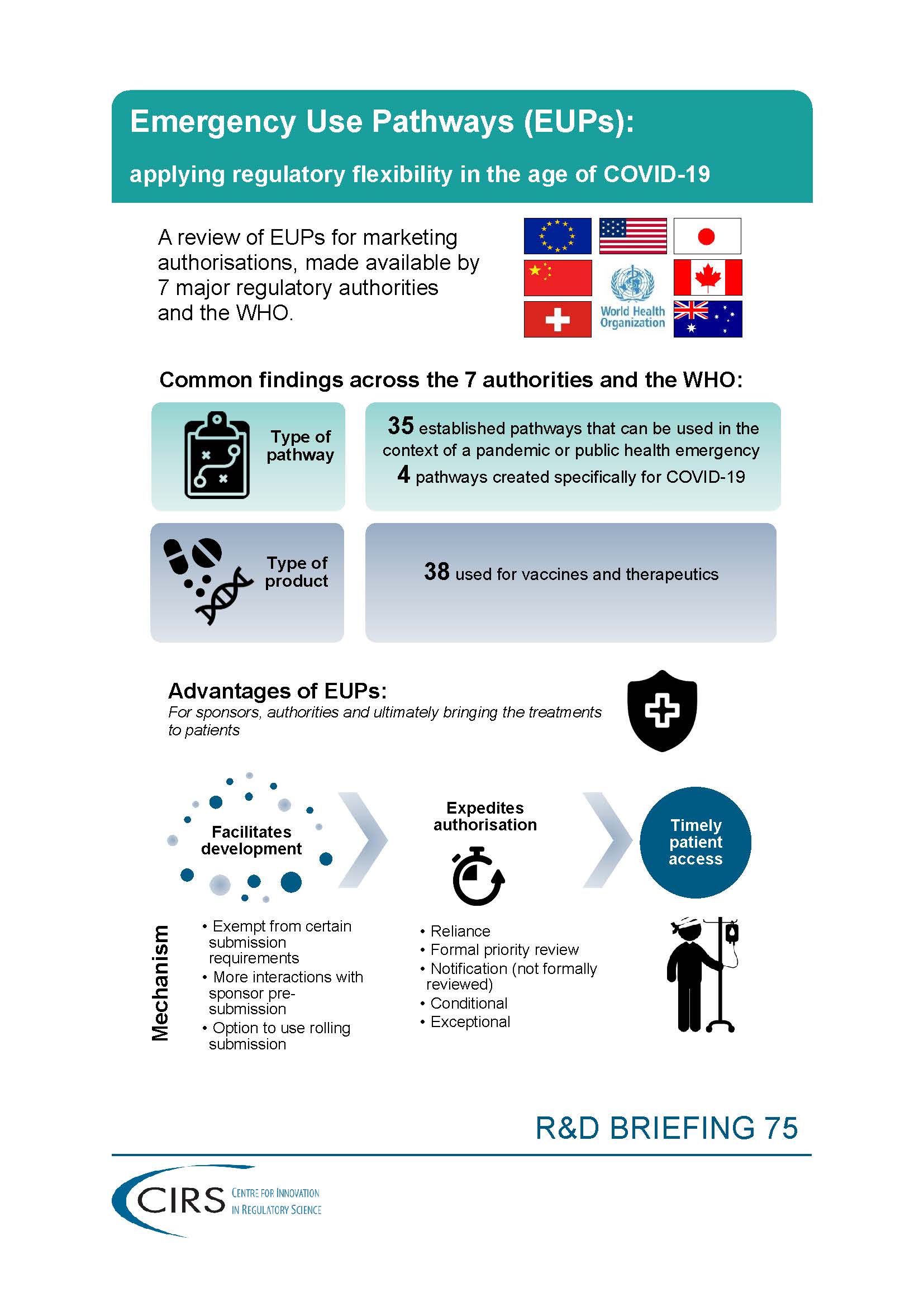
It has become clear that agencies have a number of pathways that can be used during public health emergencies for the authorisation of therapeutics and vaccines. Some of these so-called Emergency Use Pathways (EUPs) have been in place and are broadly applicable to making vaccines and other therapies available during a time of crisis, while others have been newly designed specifically in response to COVID-19.
Because some of these are less well understood by the broader scientific community, we felt that it would be helpful to provide a high-level overview to illustrate the numbers and basic characteristics of EUPs used by several key agencies.
The goal of this R&D Briefing is to illustrate, with regard to authorisation, that many EUPs are available and that regulatory flexibility is one of the most important considerations for addressing critical, unique needs.
If you have any questions or comments on this Briefing, please don’t hesitate to get in touch with us at cirs@cirsci.org.