Publications
CIRS publishes insights from its research and meetings in several forms:
- R&D Briefings – research papers produced by the CIRS team e.g. annual regulatory and HTA benchmarking briefings
- Journal articles – peer reviewed academic research papers
- Reports – from CIRS workshops and externally commissioned research projects, as well as CIRS Annual Reports
- Books – research theses from CIRS-supported PhD students
- Posters – presented at external conferences
Keep up-to-date with CIRS publications and activities by signing up to our mailing list or following CIRS on LinkedIn.
Keyter et al 2020 – A proposed regulatory review model to support SAHPRA
Background: National regulatory agencies of various sizes and maturity levels, including the South African Health Products Regulatory Authority (SAHPRA), have had to revise systems and re-engineer processes in order to …
2019 Workshop report – Optimising the regulatory review process by evaluating performance and addressing good reliance practices
This workshop was part of a series of workshops on reliance, which continues to be an important area of focus for CIRS. It builds on the outcomes of a workshop …
2019 Workshop report – Identifying and understanding uncertainty during development
This workshop was a follow-on from a collaborative forum held in Utrecht in 2018 entitled What new research can enable a joint approach by regulatory and HTA agencies to manage …
Liberti et al 2020 – Evaluation of the Caribbean Regulatory System centralised assessment process
Background: The Caribbean Regulatory System is a centralized medicine assessment procedure established to serve the needs of the Member States of the CARICOM region. In order to better understand the …
Wang et al 2020 – Benchmarking HTA agencies: methodological challenges and recommendations
Objectives: The objectives of the study were to establish a benchmarking tool to collect metrics to enable increased clarity regarding the differences and similarities across health technology assessment (HTA) agencies, …
Roadmap for Regulatory Performance
Every regulatory authority in the world has an ambition to improve its performance. In order to achieve this, agencies often establish performance indicators which they use for measuring and monitoring …
CIRS 2019 Annual Report
We’re delighted to present the inaugural CIRS Annual Report, which provides a summary of projects and workshops undertaken in 2019, as well as a historical perspective of CIRS achievements over …
An independent perspective on the East African Community Medicines Regulatory Harmonisation initiative
African regulators are taking bold transformative steps to optimise the effective and efficient use of their agency resources to assure access to quality, safe and effective medicines. In particular, the …
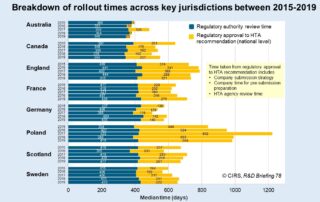
CIRS RD Briefing 78 – HTA outcomes in Australia, Canada and Europe 2015-2019
This Briefing presents data from HTADock, an ongoing metrics study that collects data on new active substances (NASs) appraised by eight HTA agencies and analyses synchronisation between the regulatory decision …
Bujar et al 2020 – A Process for Evaluating Quality Decision-Making Practices
Background: The development of a medicine is not only underpinned by good science but also by Quality Decision-Making Practices (QDMPs). Indeed, it is important to ensure that all organisations involved …
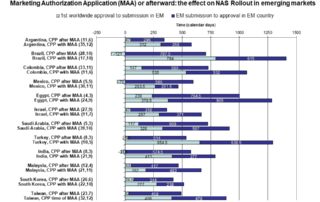
Rodier et al 2020 – Use of the CPP in 18 maturing markets
Background: The certificate of pharmaceutical product (CPP) was implemented to accelerate the availability of new drugs in developing countries by providing evidence of the quality of products and reducing the …
Keyter et al 2020 – Can standardisation of the Public Assessment Report improve benefit-risk communication?
Background: National regulatory authorities (NRAs) make the decision to register a medicine based on an assessment of its benefits and risks and publicly available assessment reports are used as a …