This R&D Briefing 60 summarises highlights from the Technical Forum convened by CIRS on 11 December 2015, Heathrow, UK.
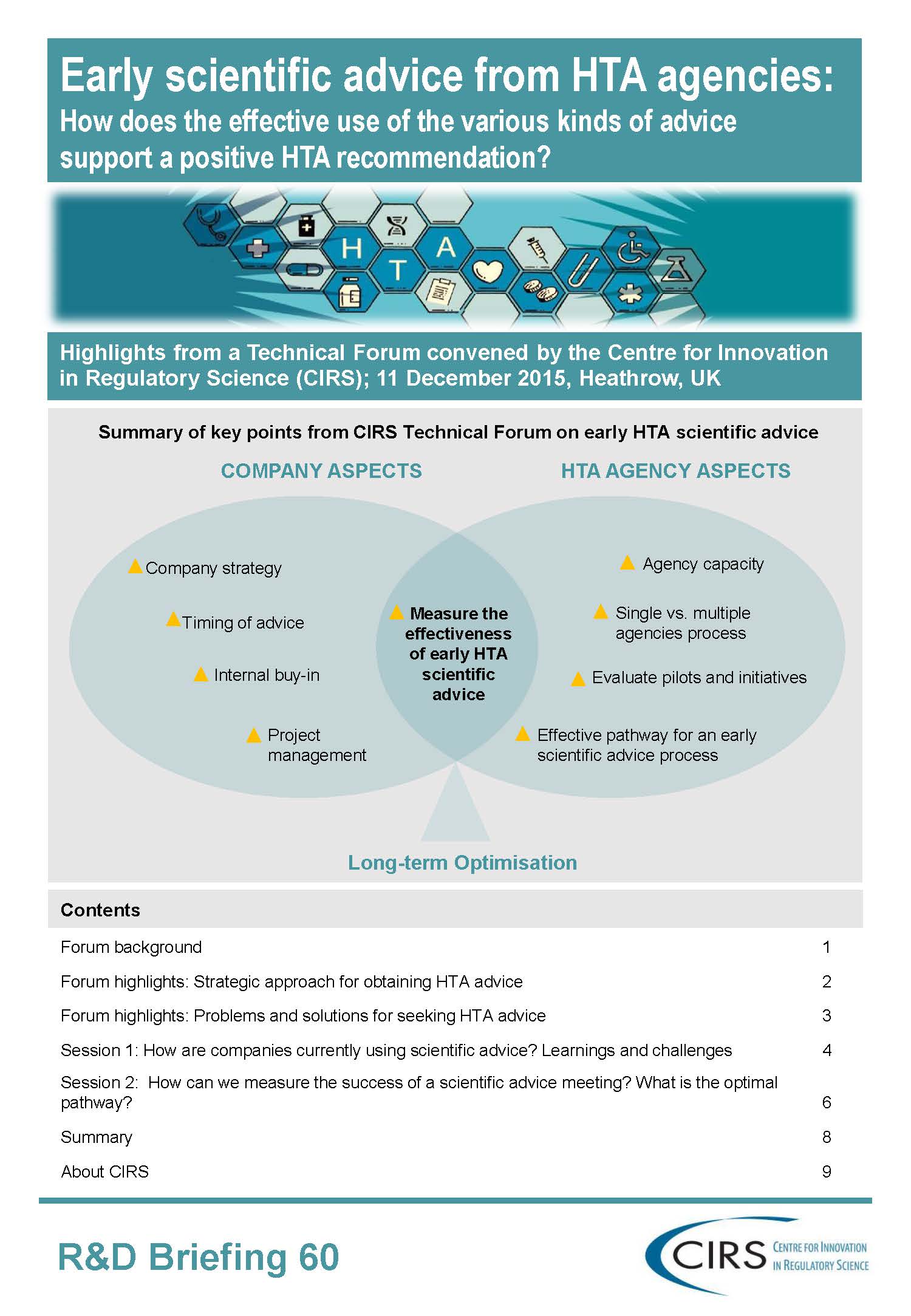
Forum objectives:
- Identify companies’ current approaches to seeking early scientific advice by discussing the outcome of the CIRS industry survey on the perception of and strategies for HTA consultations, and to discuss how this industry experience can support evidence generation and jurisdictional submissions
- Improve the understanding of the current initiatives of multi-stakeholder consultations to identify learnings and challenges posed by these pilots and to address the implications of using the pilots to achieve greater alignment of evidentiary requirements
- Discuss the optimal pathway of early HTA advice in terms of when, on what topics, and from whom to seek advice and how processes can be improved to increase the efficient use of resources.