All approved medicines have been rigorously assessed by regulatory authorities to ensure their benefits outweigh the risks. Today, pharmaceutical companies are increasingly focused on integrated global drug development strategies, with the aim of achieving worldwide registration. This approach enables them to make their new active substances (NASs) available to patients globally in a timely manner.
Since 2002, CIRS has benchmarked regulatory agencies using a methodology developed in collaboration with agencies to enable like-for-like comparisons. Published annually since 2012, our briefings review the performance of six major regulatory agencies: the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), Japan Pharmaceuticals and Medical Devices Agency (PMDA), Health Canada, Swissmedic and Australian Therapeutic Goods Administration (TGA). These briefings provide unique insights into regulatory performance, highlight areas for improvement, and support strategic planning by companies and agencies.
In recent years, there has been significant progress in reducing delays in new drug approvals in China. The introduction of expedited pathways in 2020, such as breakthrough, conditional, priority and special review pathways, has supported accelerated development and approval of drugs with significant clinical value or for urgent health needs (see CIRS R&D Briefing 84).
This study expands on the CIRS regulatory benchmarking method, exploring NASs approved between 2019-2023 by six regulatory agencies in Australia, Canada, Europe, Japan, Switzerland and the US, and assessing their regulatory status in China by January 2025. 25 NASs approved by all seven regulators were classified as ‘internationalised medicines’. This briefing focuses on the submission and approval trends of these internationalised medicines.
Key insights from the briefing:
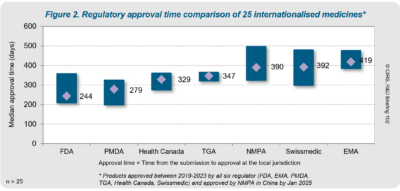
- China’s NMPA approval timelines were comparable to other agencies (median 390 days, see figure below).
- Submission strategies vary: FDA is typically first, while China is often last — though some companies are now prioritising earlier China submissions.
- 11 of the 25 internationalised NASs were submitted to all seven agencies within two to three years, while six NASs achieved full global submission within one year.
- 12 of 25 internationalised NASs received NMPA priority review; four were conditionally approved and two designated as breakthrough therapies.
- Four class 1 NASs, defined as innovative new chemical drugs not yet approved anywhere globally, were submitted to NMPA a median of 151 days ahead of their first global approval.
This briefing highlights the evolving role of China in global regulatory strategies and the importance of coordinated submissions to accelerate patient access.
Please cite this article as: Wang T, Kermad A, Bujar M & McAuslane N (2025) R&D Briefing 102: Tracking Availability in China of Medicines Approved in Six Key Global Markets. Centre for Innovation in Regulatory Science (CIRS), London, UK.
Questions?
If you have any questions or comments on this study, please get in touch with Dr Tina Wang: twang@cirsci.org.
