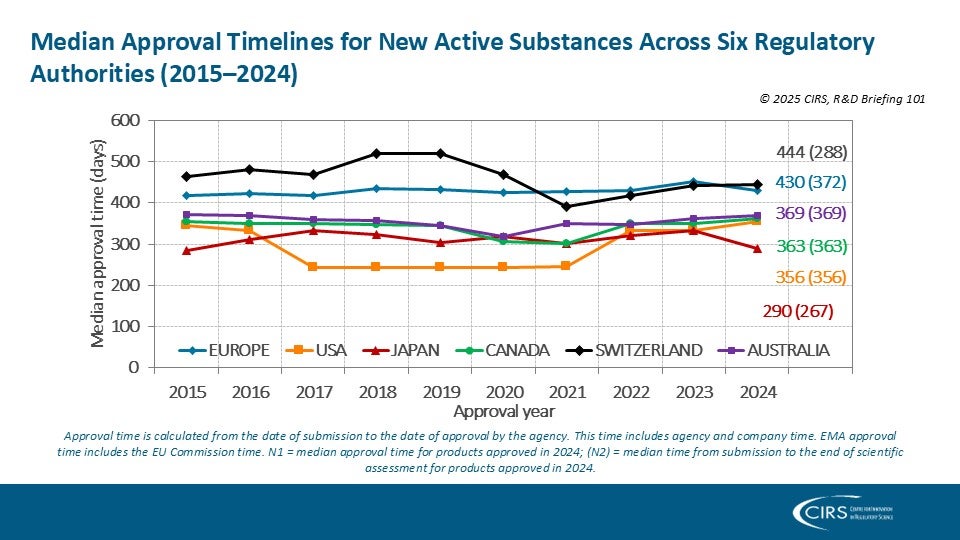
How are international regulatory organisations shaping global pharmaceutical policy? Dangy-Caye et al 2025
We’re pleased to share our latest publication in Frontiers in Medicine, developed in collaboration with Sanofi, exploring how international regulatory organisations are shaping global pharmaceutical policy. Data from our [...]