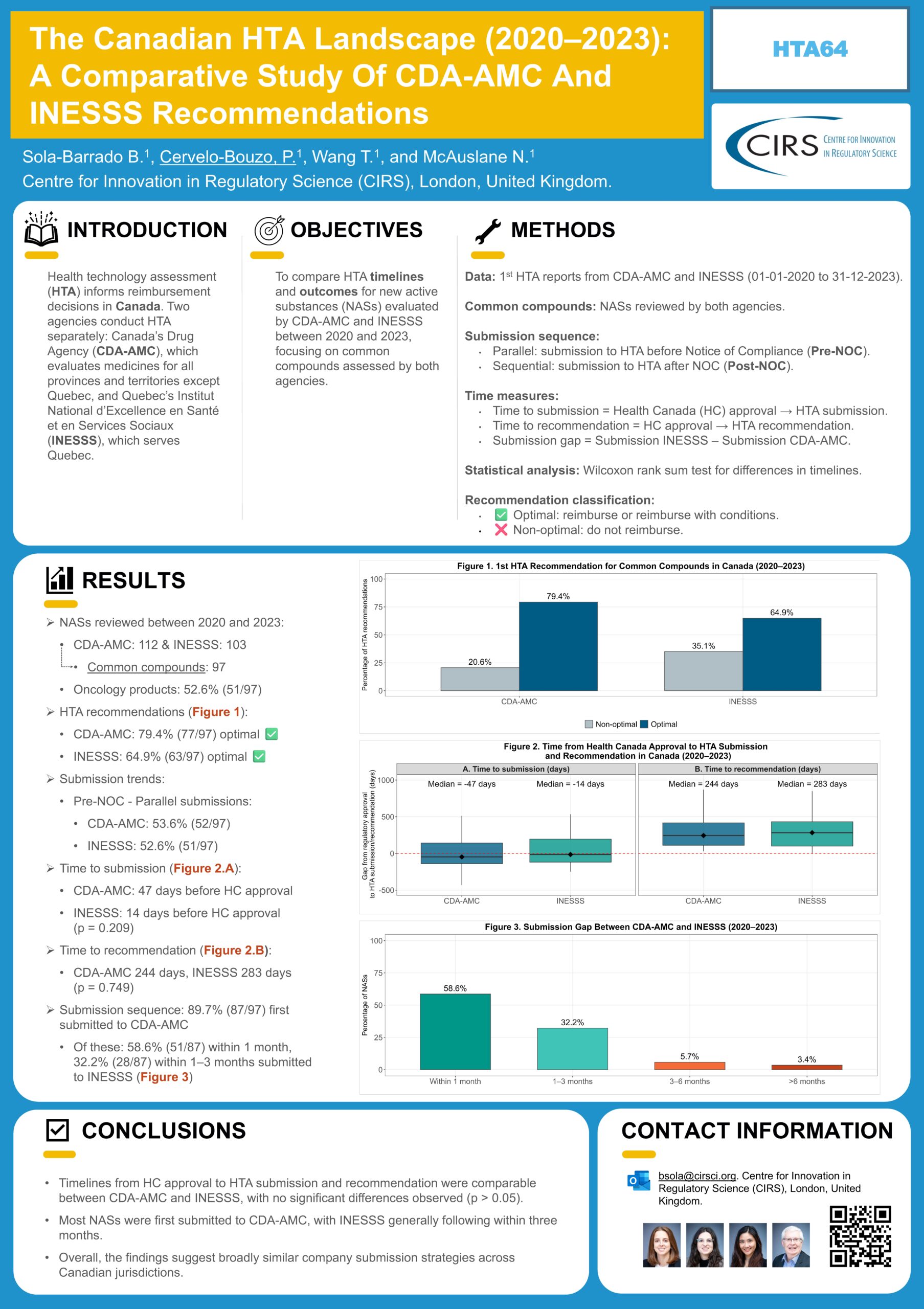
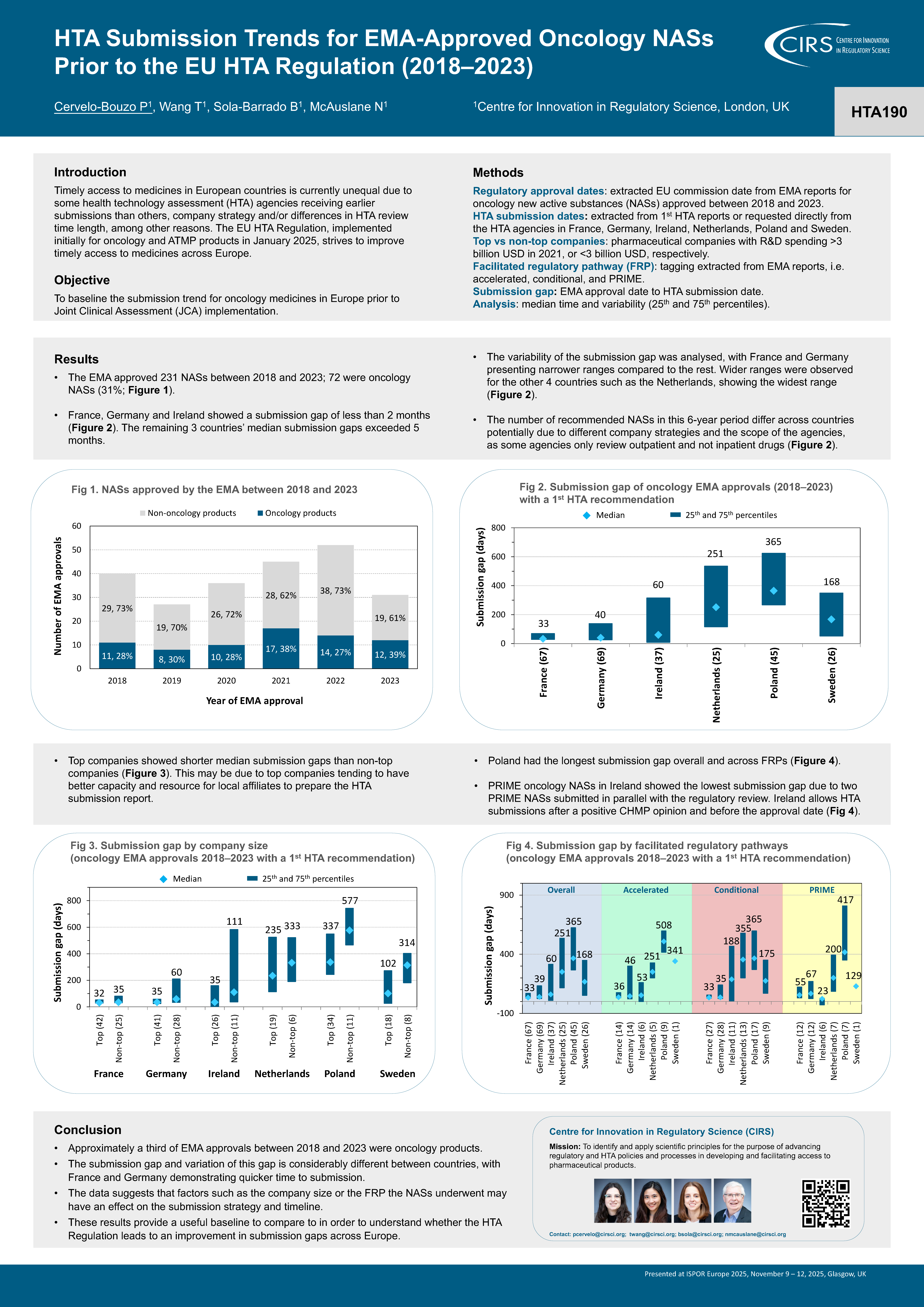
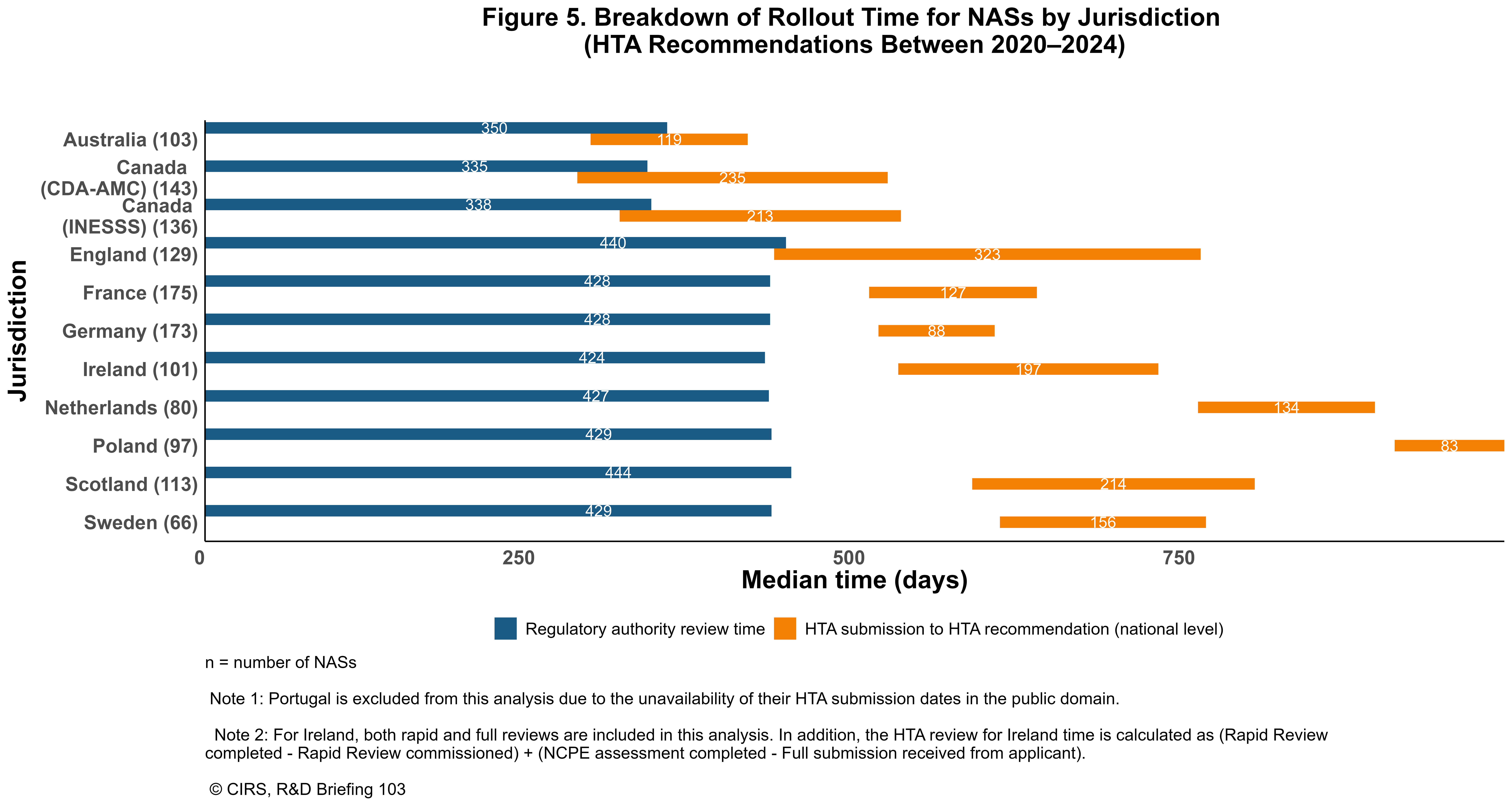
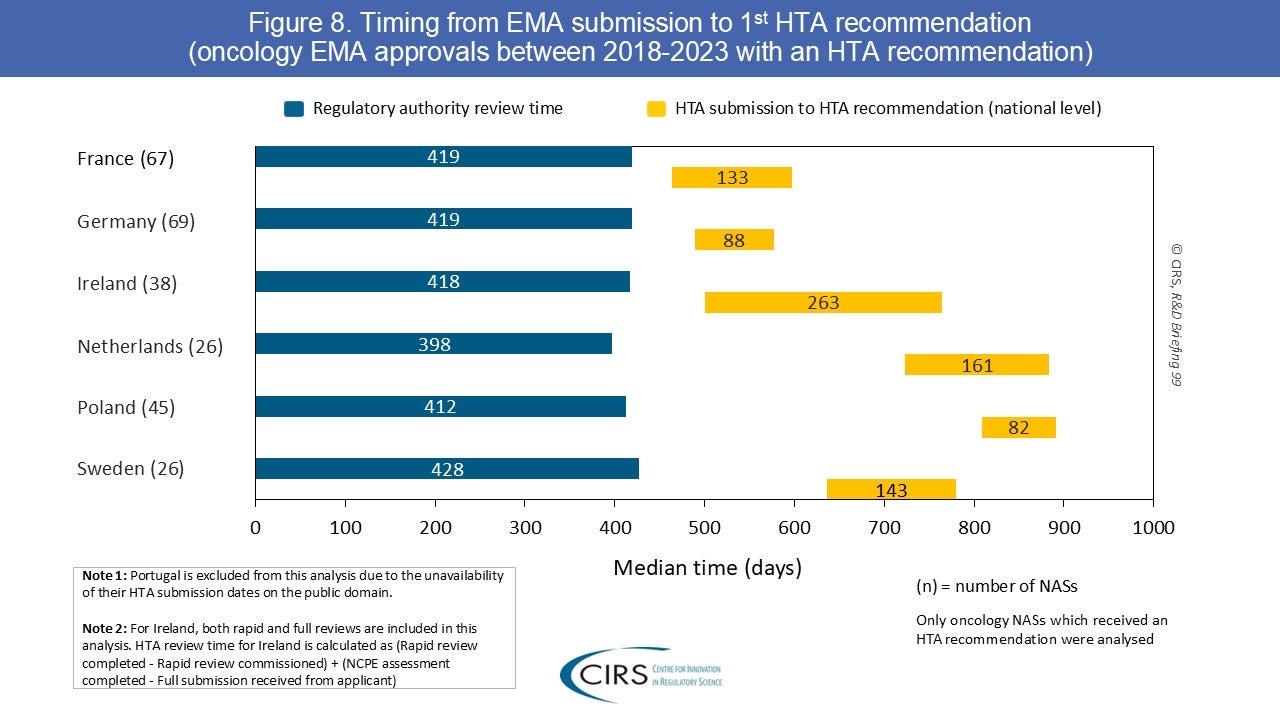
Health technology assessment (HTA)
Examining the UK HTA Landscape for Orphan Medicines
To mark Rare Disease Day on 28 February, we’re sharing findings from our work examining how orphan new active substances (NASs) are assessed by two of the UK’s HTA [...]