Mature regulatory agency benchmarking
CIRS has been benchmarking regulatory agencies since 2002 using a methodology developed with agencies that ensures like-for-like comparisons. The resulting analyses, published annually since 2012, give unique insights into regulatory processes and practices, identify where improvements can be made and inform company and agency strategies.
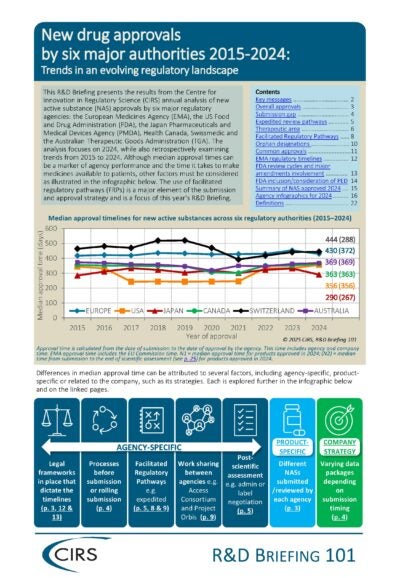
The annual benchmarking study focuses on the review performance of six regulatory agencies: the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), Japan Pharmaceuticals and Medical Devices Agency (PMDA), Health Canada, Swissmedic and Australian Therapeutic Goods Administration (TGA). Data is collected from the public domain and/or directly from the agencies, is validated by CIRS and maintained in an internal CIRS database.
The results of these benchmarking studies have been endorsed and quoted by the agencies in their annual reports and conference presentations, and have also been used by other stakeholders. For example, regulatory benchmarking data from R&D Briefing 88 was cited by the European Commission in the Draghi Report on EU Competitiveness (Part B, p193) that looks at the challenges faced by the industry and companies in the European Union. The report’s findings are aiding the Commission in developing a new strategy for Europe’s sustainable prosperity and competitiveness.
CIRS has also used its regulatory review database to probe for new insights and answer other research questions e.g. to what degree are review outcomes aligned for new active substances between the European Medicines Agency and the US Food and Drug Administration? (full study available here).